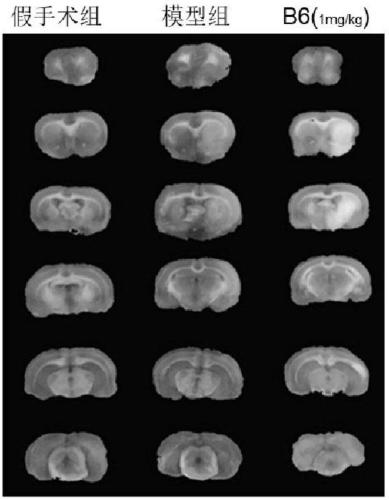
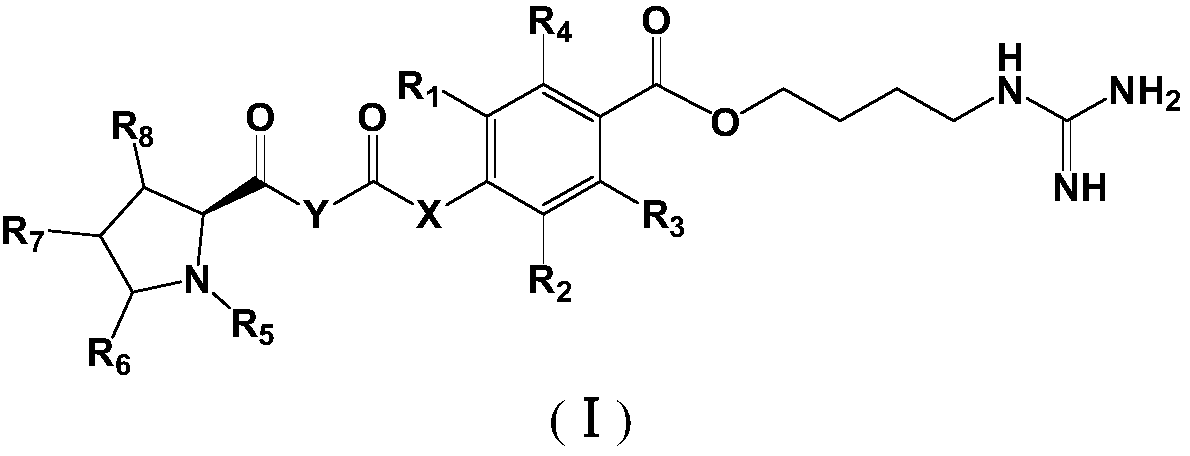
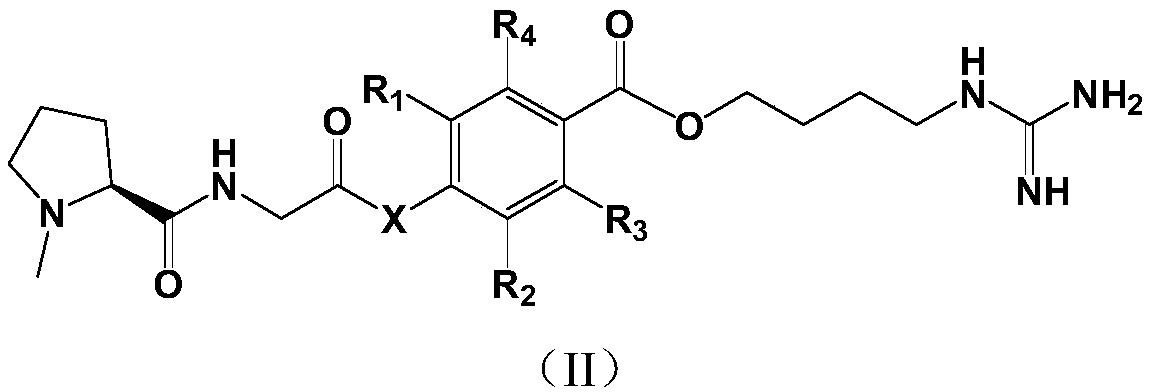
Proline derivative and application of proline derivative in preparation of medicine for treatment of cardiovascular and cerebrovascular diseases
A technology of proline and derivatives, applied in the field of medicine, can solve the problems of low bioavailability, short half-life of stachydine, poor anti-ischemic stroke activity, etc., and achieve the effect of inhibiting cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: The synthetic route of N-methyl-L-proline b is as follows:
[0049]
[0050] Dissolve L-proline (4.0g, 34.8mmol) in methanol (40mL), add 40% formaldehyde aqueous solution (2.8mL, 38.2mmol) and 10% palladium carbon (1g) successively, add hydrogen, and add hydrogen at room temperature Reacted for 24 hours. After the reaction was complete, it was filtered and the mother liquor was concentrated to dryness to obtain 4 g of white solid with a yield of 89%, which was N-methyl-L-proline (compound b). ESI-MS: (m / z, %)=130[M+H] + .
Embodiment 2
[0051] Embodiment 2: The synthetic route of compound B-4 is as follows:
[0052]
[0053] The N-methyl-L-proline b (1.29g, 10mmol) was added into dichloromethane (20mL), DCC (2.47g, 12mmol) and glycine (0.75g, 10mmol) were added successively, and reacted at room temperature for 24 hours, filtered, and the mother liquor was concentrated to obtain 1 g of white solid with a yield of 54%, namely compound B-4. 1 H NMR (500MHz, CD 3 OD)δ=4.14(t,J=8.3,1H),4.00(s,2H),3.71(ddd,J=11.4,7.7,4.1,1H),3.22(d,J=11.1,1H),2.95( d, J = 10.5, 3H), 2.58 (dd, J = 12.2, 7.5, 1H), 2.24-2.02 (m, 3H).
Embodiment 3
[0054] Embodiment 3: The synthetic route of compound B-5 is as follows:
[0055]
[0056] The B-4 (1.86g, 10mmol) was added into dichloromethane (20mL), followed by DCC (2.47g, 12mmol) and Y5 (3.1g, 10mmol), reacted at room temperature for 24 hours, filtered, and the mother liquor was concentrated to 1.4 g of colored oily substance, the yield is 30%, which is compound B-5. ESI-MS: (m / z, %)=480[M+H] + . 1 H NMR (500MHz,D 2 O) δ=7.43(s, 2H), 4.49(d, J=7.8Hz, 2H), 4.42(t, J=6.5Hz, 2H), 4.31(t, J=8.5Hz, 1H), 3.92(s ,6H),3.85-3.80(m,1H),3.31-3.25(m,3H),2.99(s,3H),2.68-2.61(m,1H),2.32–2.04(m,3H),1.94-1.84 (m,2H), 1.80–1.73(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com