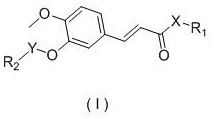
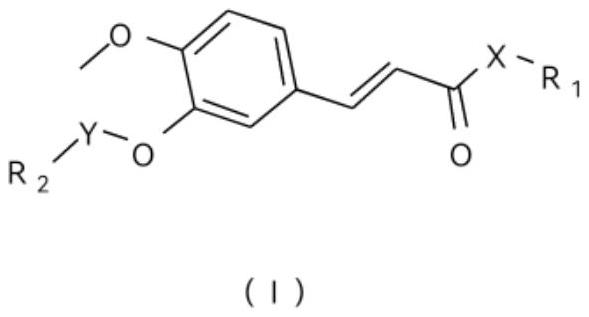
Ferulic acid eugenol and isoeugenol heterozygote and application
A technology of isoeugenol and eugenol, which is applied in the fields of application, chemicals for biological control, biocides, etc., can solve the problem of inactivated virus activity, single structure, and anti-plant virus activity, which only include treatment and protection issues, to achieve the effect of low production cost, simple preparation process and excellent treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The preparation method of ferulic acid eugenol hybrid A1 comprises the steps:
[0117] (1) preparation of methyl ferulate:
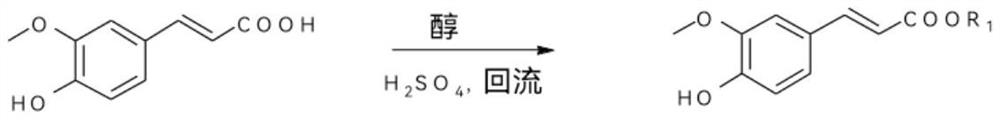
[0118] Get ferulic acid (10g, 51.50mmol) in a three-necked flask, add 50mL of methanol, stir for 10 minutes, then add dropwise 98% concentrated sulfuric acid (5.06g, 51.50mmol) and react at a temperature of 80°C for about 8 hours. The solvent was recovered under pressure, 10 mL of water was added to the system, and the pH was adjusted to 8 with saturated sodium bicarbonate solution, and ethyl acetate was added to extract three times (100 mL each time), the extracts were combined, dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure to obtain 9.85g of methyl ferulic acid intermediate, yield 91.7%.
[0119] (2) Preparation of bromoethyl ferulic acid methyl ester:
[0120] Take methyl ferulate (5.0g, 24.01mmol) in a three-necked flask, then add 50mL of n-butyl ketone and anhydrous potassium carbonate (3.98g, 28....
Embodiment 2
[0124] The preparation method of ferulic acid isoeugenol hybrid A2 comprises the steps:
[0125] Step (1)~(2) is the same as embodiment 1
[0126] (3) Preparation of target compound A2:
[0127] Take isoeugenol (1.5g, 9.13mmol) in a three-necked flask, then add 20mL of acetonitrile and anhydrous potassium carbonate (1.51g, 10.96mmol), stir at room temperature for 1 hour, then add bromoethyl ferulic acid methyl ester ( 2.88g, 9.13mmol), and reacted at 80°C for about 4 hours, filtered, the filtrate was concentrated under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain the target compound A2, 2.95g, yield 81.2%.
Embodiment 3
[0129] The preparation method of ferulic acid eugenol hybrid A3 comprises the steps:
[0130] (1) preparation of ethyl ferulate:
[0131] Get ferulic acid (10g, 51.50mmol) in a three-necked flask, add 50mL of ethanol, stir for 10 minutes, then add dropwise 98% concentrated sulfuric acid (5.06g, 51.50mol) and react at a temperature of 80°C for about 8 hours. The solvent was recovered under pressure, water was added to the system, and the pH was adjusted to 8 with saturated sodium bicarbonate solution, then ethyl acetate was added for extraction three times (100 mL each time), the extracts were combined, dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure to obtain 10.50 g of ethyl ferulic acid intermediate, yield 91.7%.
[0132] (2) Preparation of bromoethyl ferulic acid ethyl ester:
[0133] Take ethyl ferulate (5.0g, 22.50mmol) in a three-necked flask, then add 50mL of n-butyl ketone and anhydrous potassium carbonate (3.73g, 27.00mmol),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com