Activity evaluation method for Fe-based ft (Fischer-Tropsch) synthesis catalyst
A technology for Fischer-Tropsch synthesis and Tropsch synthesis, which is applied in the direction of chemical analysis using catalysis, and can solve the problem of inability to quickly and effectively determine the deactivation boundary of iron-based Fischer-Tropsch synthesis catalysts.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
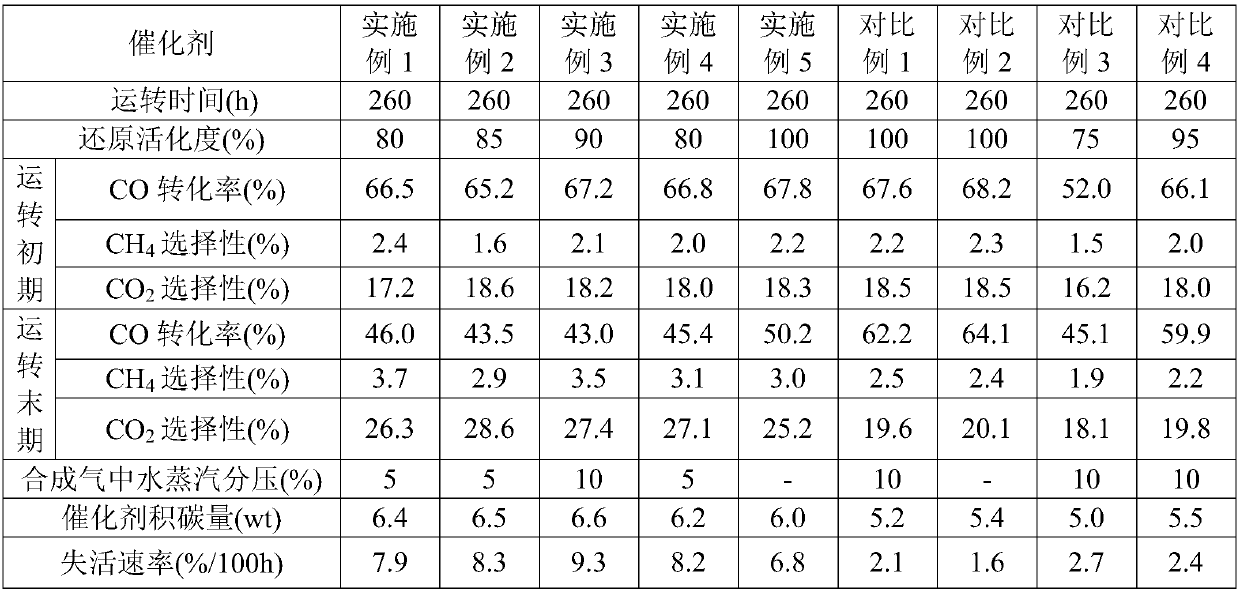
[0067] The Fischer-Tropsch synthesis catalyst of 10g oxidized state is mixed with 300mL molten paraffin evenly to make a slurry, filled in a stirred tank reactor, first passed into an inert gas Ar for purging, and then introduced a reducing gas (H 2 and CO syngas, where the H in the syngas 2 The volume ratio of CO and CO is 20:1); adjust the pressure of the stirred tank reactor to the reduction and activation pressure of 0.5MPa, and control the space velocity of the reduction gas to 6000h -1 , first raise the temperature inside the stirred tank reactor from 30°C to 200°C at a heating rate of 40-45°C / h, and then raise the temperature inside the stirred tank reactor from 200°C at a heating rate of 5-10°C / h To 260°C, reduce and activate for 3 hours, and then introduce reducing gas containing inert gas Ar, wherein the volume percentage of inert gas Ar is 10%, and the rest is reducing gas.
[0068] The extent to which reductive activation proceeded was determined by measuring the ...
Embodiment 2
[0072] Mix 2g of oxidized Fischer-Tropsch synthesis catalyst with 10mL of quartz sand evenly, fill it into a fixed-bed reactor, and first pass inert gas N 2 Purge, then introduce reducing gas (H 2 and CO syngas, where the H in the syngas 2 The volume ratio of CO and CO is 30:1); adjust the pressure of the fixed bed microreactor to the reduction and activation pressure of 2.5MPa, and control the space velocity of the reduction gas to 6200h -1 , first raise the temperature inside the fixed-bed microreactor from 30°C to 200°C at a heating rate of 40-45°C / h, and then raise the temperature inside the fixed-bed microreactor from 200°C at a heating rate of 5-10°C / h ℃ to 260 ℃, reduction and activation for 4h, and then introduce inert gas containing N 2 The reducing gas, inert gas N 2 The volume percentage is 15%, and the rest is reducing gas.
[0073] The extent of the reduction activation reaction was determined using silica gel to absorb the moisture in the tail gas. When the ...
Embodiment 3
[0077] The Fischer-Tropsch synthesis catalyst of 10g oxidized state is mixed with 300mL molten paraffin evenly to make a slurry, filled in a stirred tank reactor, first passed into an inert gas Ar for purging, and then introduced a reducing gas (H 2 and CO syngas, where the H in the syngas 2 The volume ratio of CO and CO is 50:1); adjust the pressure of the stirred tank reactor to the reduction and activation pressure of 3MPa, and control the space velocity of the reduction gas to 8000h -1 , first raise the temperature inside the stirred tank reactor from 30°C to 200°C at a heating rate of 40-45°C / h, and then raise the temperature inside the fluidized bed reactor from 200°C at a heating rate of 5-10°C / h To 280°C, reduction and activation for 3 hours, and then introduce reducing gas H containing inert gas Ar 2 and CO synthesis gas, in which the volume percentage of inert gas Ar is 10%, and the rest is reducing gas H 2 and CO synthesis gas.
[0078] The extent to which reduct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com