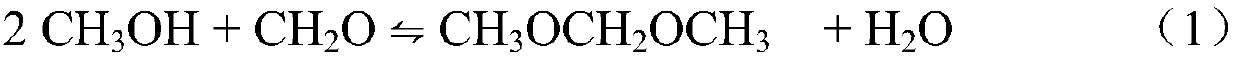Continuous catalytic extraction method for preparing methylal
A technology of methylal and formaldehyde, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as separation difficulties and achieve the effect of easy separation of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
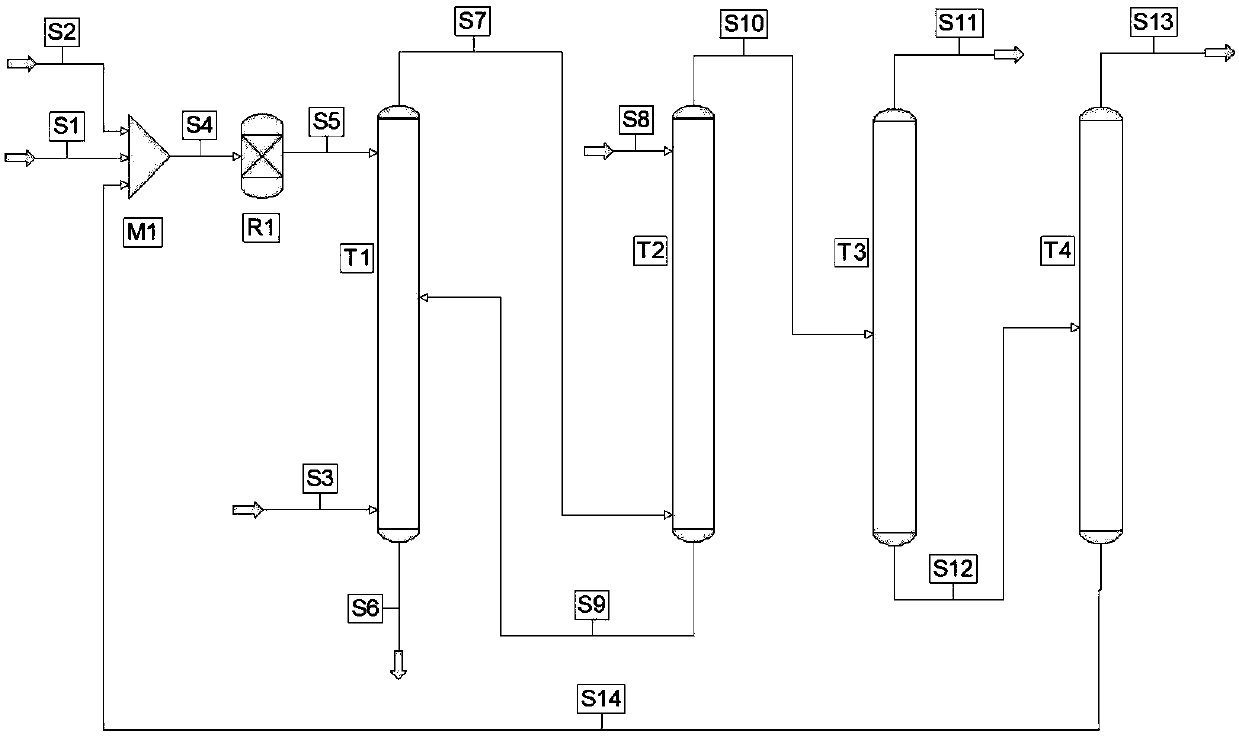
[0087]The prereactor is a stainless steel fixed bed reactor with an inner diameter of 14mm and a height of 0.3m, filled with 40 ml of strong acidic cation exchange resin catalyst, and the outlet of the prereactor is connected to the heavy phase feed pipe of the catalytic extraction reactor.
[0088] A stainless steel catalytic extraction reactor with an inner diameter of 20mm and a height of 1.3m is filled with 160ml of strongly acidic cation exchange resin catalyst and 200ml of Φ3×3mm stainless steel θ mesh ring, which are evenly mixed. The filling height is 1.2m, and the top 10cm is an empty tube. Set the heavy phase feed pipe at the top of the reactor 1.2m, that is, the top of the catalyst and θ mesh ring bed, set the light phase discharge pipe on the top of the reactor to connect the back pressure valve to control the reaction pressure, and set the light phase feed pipe at the bottom of the reactor , the bottom of the reactor is connected to a temperature-resistant and pres...
Embodiment 2
[0093] Adopt the same prereactor, catalytic extraction reactor, catalyst, catalyst loading method and loading amount as in Example 1.
[0094] 38wt% formaldehyde solution (containing methanol 3wt%), methanol and PODE 2+ (PODE 2 Accounting for 95wt%, PODE 3+ Accounting for 4wt%) enters the prereactor after mixing, and feed rate is respectively 10ml / min, 11ml / min, 0.8ml / min; Alkanes each account for 20wt%) as the extractant, feed from the bottom of the reactor, feed rate 20ml / min; pre-reactor reaction temperature 60 ℃, catalytic extraction reactor reaction temperature 80 ℃, reaction pressure 0.5MPa.
[0095] The output of the light phase at the top of the reactor is 24.7g / min, including 0.13wt% formaldehyde, 0.58wt% methanol, 0.12wt% water, 41.5wt% DMM, PODE 2+ 2.7wt%, and the rest are alkane mixtures and trace by-products.
[0096] The output of the heavy phase at the bottom of the reactor is 9.9g / min, including 0.8wt% methanol, 0.01wt% formaldehyde, 0.06wt% alkane mixture...
Embodiment 3
[0098] Adopt the same prereactor, catalytic extraction reactor, catalyst, catalyst loading method and loading amount as in Example 1.
[0099] 38wt% formaldehyde solution (containing methanol 3wt%), methanol and PODE 2+ (PODE 2 Accounting for 95wt%, PODE 3+ accounted for 4wt%) into the pre-reactor after mixing, the feed rate was 8ml / min, 8.8ml / min, 1ml / min; using toluene and ethylbenzene mixture (weight ratio 1:1) as the extractant, from the bottom of the reactor Feed, the feed rate is 6.3ml / min; the reaction temperature of the pre-reactor is 70°C, the reaction temperature of the catalytic extraction reactor is 60°C, and the reaction pressure is 0.5MPa.
[0100] The output of the light phase at the top of the reactor is 14.8g / min, including 0.18wt% formaldehyde, 0.8wt% methanol, 0.06wt% water, 56.2wt% DMM, PODE 2+ 5.8wt%, and the rest are toluene, ethylbenzene and trace by-products.
[0101] The output of the heavy phase at the bottom of the reactor is 8 g / min, including ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com