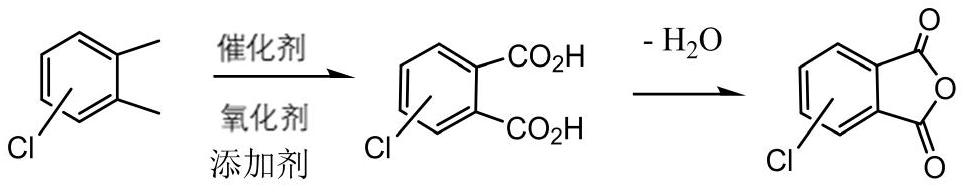
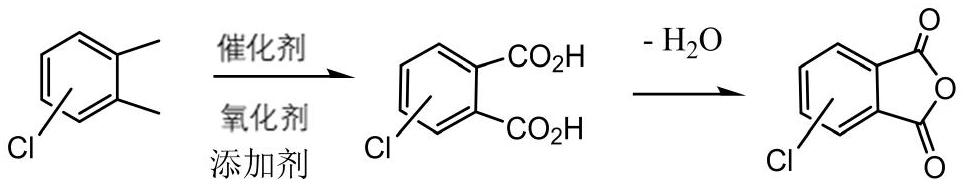
Method for preparing chlorophthalic anhydride through solvent-free liquid-phase catalytic oxidation
A chlorophthalic anhydride, liquid-phase catalysis technology, applied in the field of synthetic chemistry, can solve problems such as reducing investment in fixed assets, and achieve the effects of easy product separation and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 500mL glass reaction tube, add 140g 4-chloro-o-xylene (1moL), catalyst cobalt acetate 0.3g, manganese acetate 0.1g, tetrabromoethane 1g, 4-chlorophthalic acid 10g, mix, stir and heat To 150°C, start to feed oxygen with a purity of 100%, the flow rate is 360mL / min, and the temperature is controlled at 200°C for 10 hours. When the oxygen concentration in the tail gas is detected to be 95%, the reaction is stopped, and then distilled and dehydrated to obtain 4-chlorophthalic anhydride 182.3 g, yield 94%, melting point 96°C.
Embodiment 2
[0018] In a 500mL glass reaction tube, add 140g of 3-chloro-o-xylene (1moL), 0.4g of catalyst cobalt acetate, 0.15g of manganese acetate, 1.5g of tetrabromoethane, and 13g of 3-chlorophthalic acid, mix and stir Heat to 150°C, start to feed oxygen with a purity of 100%, flow rate 240mL / min, start the temperature, control the temperature at 210°C for 15 hours, stop the reaction when the concentration of oxygen in the tail gas is detected to be 95%, and then distill and dehydrate to obtain 3-chloro Phthalic anhydride 181.3g, yield 93%, melting point 125°C.
Embodiment 3
[0020] In a 500mL glass reaction tube, add 14g mixed 3-, 4-chloro-o-xylene mixture (0.1moL, 0.45 / 0.55), catalyst cobalt acetate 0.04g, manganese acetate 0.015g, sodium bromide 0.3g, 3-chloro Mix 1.3 g of substituted phthalic acid, stir and heat to 150°C, start to feed air at a flow rate of 240mL / min, gradually raise the temperature to 210°C, and react for 8 hours, stop the reaction when the concentration of oxygen in the tail gas is detected to be 20%, and then Distillation and dehydration gave 180.3 g of a mixture of 3- and 4-chlorophthalic anhydride, with a yield of 92% and a melting point of 98°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com