Methods for treating disease using inhibitors of bone morphogenetic protein 6 (BMP6)
A technology of ferritin and hemoglobin, which is used in anti-growth factor immunoglobulin, blood diseases, disease diagnosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
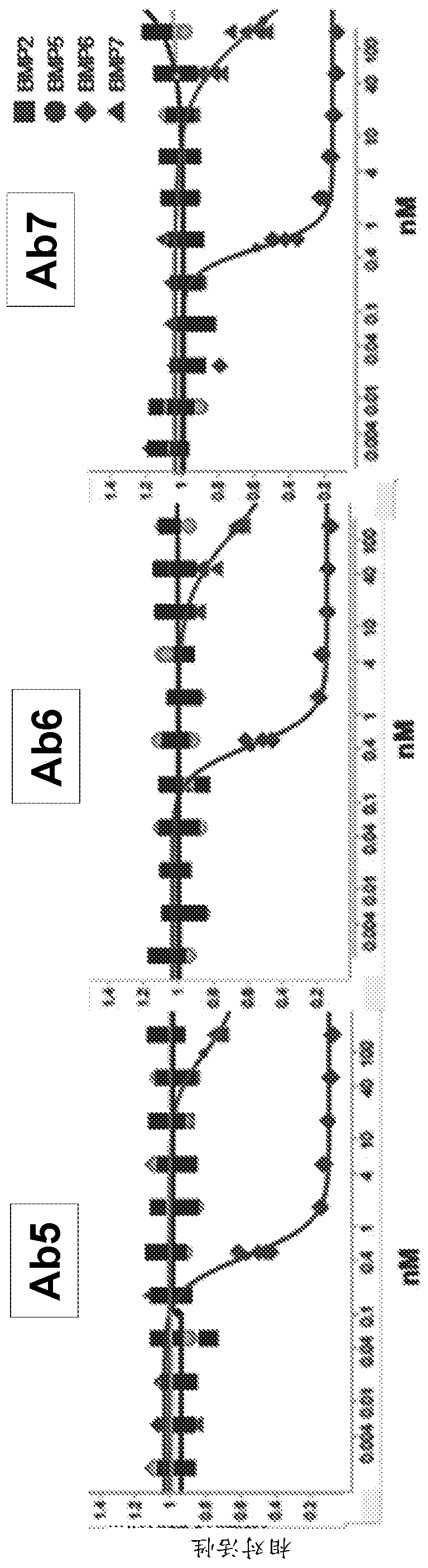
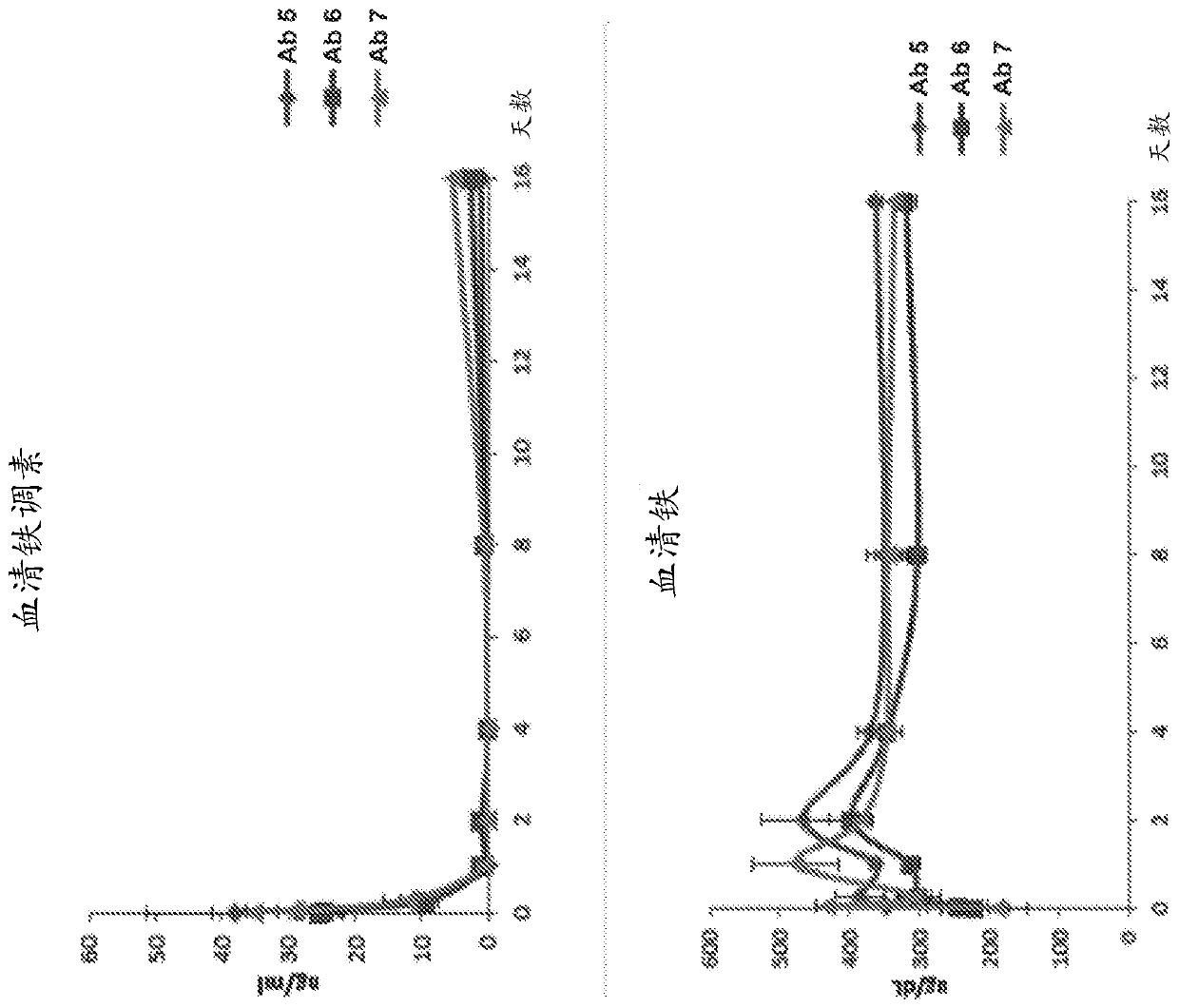
[0620] Example 1 - In vitro and in vivo activity and PK / PD of anti-BMP6 antibodies
[0621] Material
[0622] Test compounds were antibodies 5, 6 and 7 at a concentration of approximately 8 mg / ml in 50 mM citrate buffer, pH 7.0, 150 mM NaCl (Table 8) and diluted in PBS prior to animal dosing. Male C56BL / 6 mice or Sprague Dawley rats were used (Table 9).
[0623] Table 8. Characteristics of BMP6 antagonist antibodies
[0624]
[0625]
[0626] Table 9. Animal Characteristics
[0627]
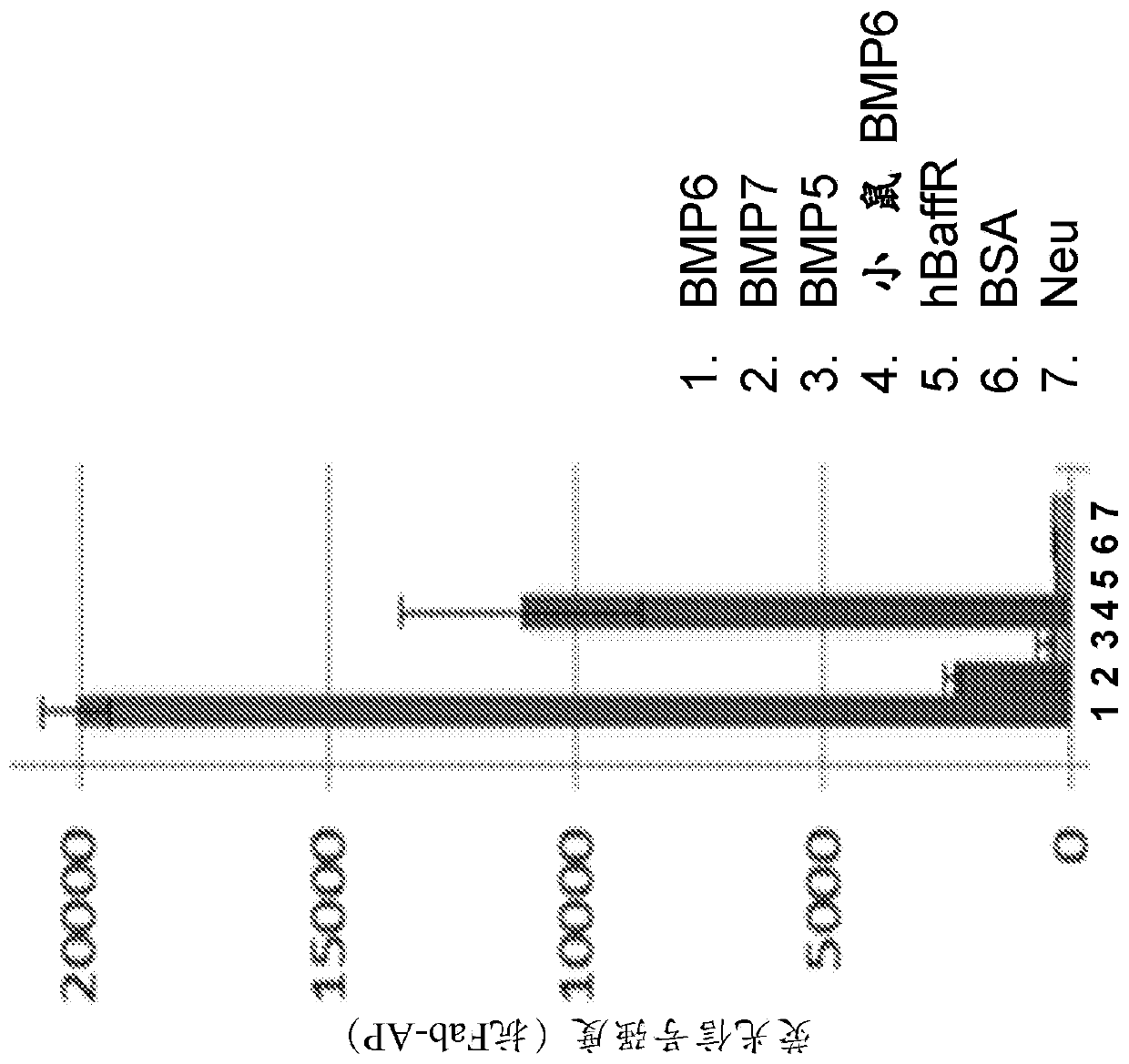
[0628] For the BMP reporter assay, a lentiviral vector containing the BMP response element BRE in the promoter was constructed [Korchynskyi et al. 2002. J. Biol. Chem. 277:4882-91], thereby driving - Firefly luciferase from BRE2-Luc2. Lentiviral vectors were used for stable transfection of the HEP3B hepatoma cell line. Cell lines were maintained in EMEM containing 10% fetal bovine serum, 1% penicillin / streptomycin and 5ug / ml blasticidin. Recombinant human BMP protein was purchased ...
example 2
[0667] Example 2 - Clinical Program for Testing BMP6 Antibodies in Humans Clinical Trial Program: Evaluation of therapies using antibodies or antigen-binding fragments thereof that bind human BMP6.
[0668]Patients with end-stage renal disease (ESRD) produce little, if any, erythropoietin (EPO) and often require regular administration of exogenous EPO and intravenous (IV) infusion of iron to achieve EPO-induced Hgb synthesis . Up to one-third of chronic hemodialysis (HD) patients do not respond adequately to EPO, primarily due to intracellular sequestration of iron. Hepcidin is primarily eliminated by the kidneys, with insufficient clearance by dialysis. Thus, chronic HD patients tend to have significantly elevated levels of hepcidin, which blocks the mobilization of iron for erythropoiesis. Once body iron stores reach critical levels (indicated by high serum ferritin levels), IV iron therapy is no longer effective or recommended. Current guidelines advise against administe...
example 3
[0836] Example 3: TSAT levels in patients treated with 0.01 mg / kg Antibody 7
[0837] Data including TSAT (iron saturation, %) levels were evaluated for the first 10 patients treated according to the clinical protocol described in Example 2, where each patient received a single infusion of Antibody 7 at 0.01 mg / kg. None of these patients showed any hepatic safety signal defining a no-observed adverse effect level (NOAEL) of 0.1 mg / kg / week. Cohort 1 included 5 anemic hemodialysis patients with low ferritin levels less than or equal to 500 ng / mL, while cohort 2 included 5 anemic hemodialysis patients with higher ferritin levels (500 to 1000 ng / mL).
[0838] Among the five Cohort 1 (low ferritin) patients receiving 0.01 mg / kg of Antibody 7, post-dose TSAT levels increased by an average of only 9.8% (average post-dose 38.6% vs. pre-dose 24.8%). In contrast, among the five Cohort 2 (high ferritin) patients receiving 0.01 mg / kg Antibody 7, TSAT levels increased by an average of 17....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com