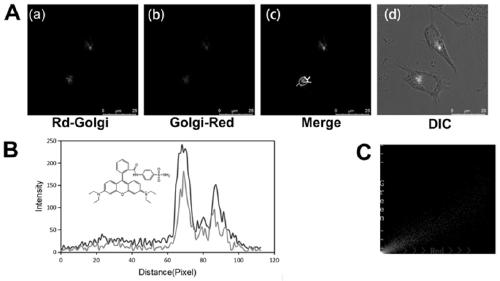
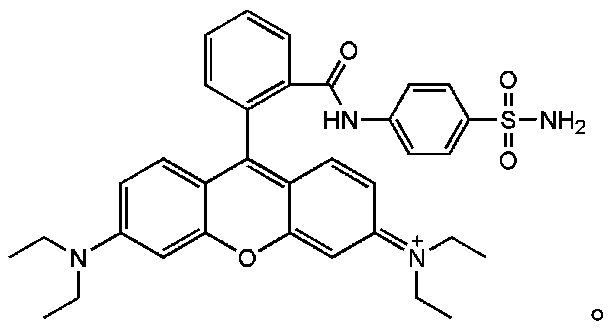
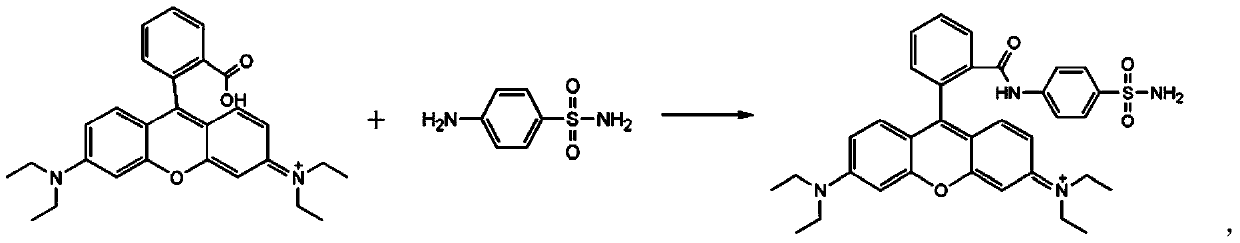
Compound represented by formula (I), and preparation method and application thereof
A compound and reaction technology, applied in the field of formula compounds and their preparation, can solve the problems of complex synthesis, high price, technology monopoly, etc., and achieve the effect of cheap and easy-to-obtain raw materials and simple synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of dye Rd-Golgi:
[0036] The raw material Rhodamine B (0.48g) was dissolved in 2.5mL of phosphorus oxychloride solution and refluxed at 80°C for 12h. After the reaction was completed, excess phosphorus oxychloride was removed by rotary evaporation. Then dissolve the product of the above step with 10mL acetonitrile, and add sulfonamide (0.4305g) and 324μL triethylamine as an acid-binding agent, react at room temperature for 12h, need to use triethylamine as an acid-binding agent, after the acid chloride and sulfonamide react during the reaction Hydrogen bromide will be generated, adding triethylamine can neutralize hydrogen bromide and move the chemical reaction equilibrium forward. Then using dichloromethane:methanol=20:1 as eluent, the compound was purified by column chromatography to obtain a pink solid named as Rd-Golgi (the yield was 21%).
[0037] NMR and mass spectrometry characterization:
[0038] 1 H NMR (400MHz, CDCl 3 )δ=7.91(dd,J=6.2,1.7,1H),7...
Embodiment 2
[0042]Synthesis of dye Rd-Golgi:
[0043] The raw material rhodamine B (0.51g) was dissolved in 2.5mL of thionyl chloride solution and refluxed at 85°C for 14h. After the reaction was completed, excess phosphorus oxychloride was removed by rotary evaporation. Then the product of the above step was dissolved with 10 mL of acetonitrile, and sulfonamide (0.4305 g) and 324 μm triethylamine were added as acid-binding agents, and reacted at room temperature for 12 h. Then, dichloromethane:methanol=20:1 was used as eluent, and the compound was purified by column chromatography to obtain a pink solid.
Embodiment 3
[0045] Synthesis of dye Rd-Golgi:
[0046] The raw material Rhodamine B (0.45g) was dissolved in 2.5mL of phosphorus oxychloride solution and refluxed at 78°C for 12h. After the reaction was completed, excess phosphorus oxychloride was removed by rotary evaporation. Then the product of the above step was dissolved in 10 mL of acetonitrile, and sulfonamide (0.4214 g) and 324 μL of triethylamine were added as an acid-binding agent, and reacted at room temperature for 12 h. Then, dichloromethane:methanol=20:1 was used as eluent, and the compound was purified by column chromatography to obtain a pink solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com