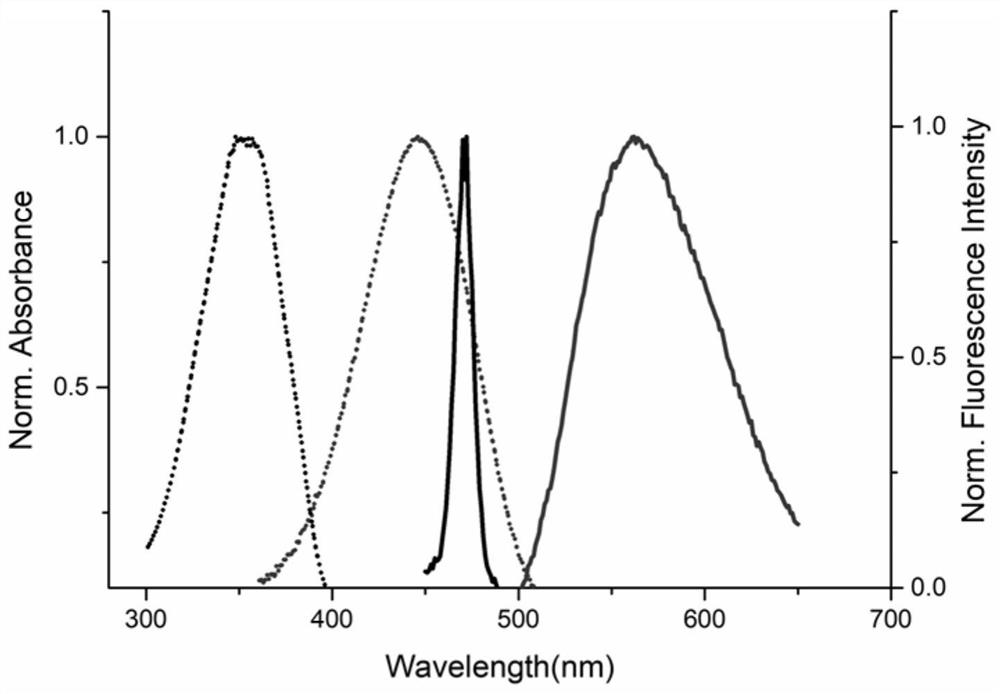
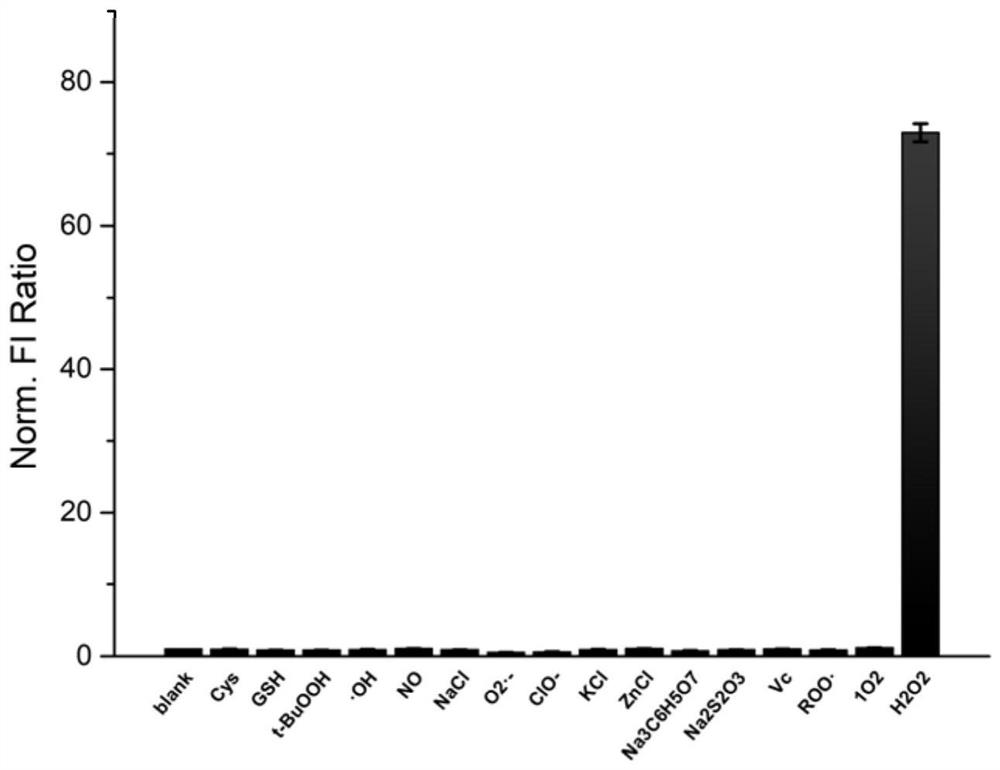
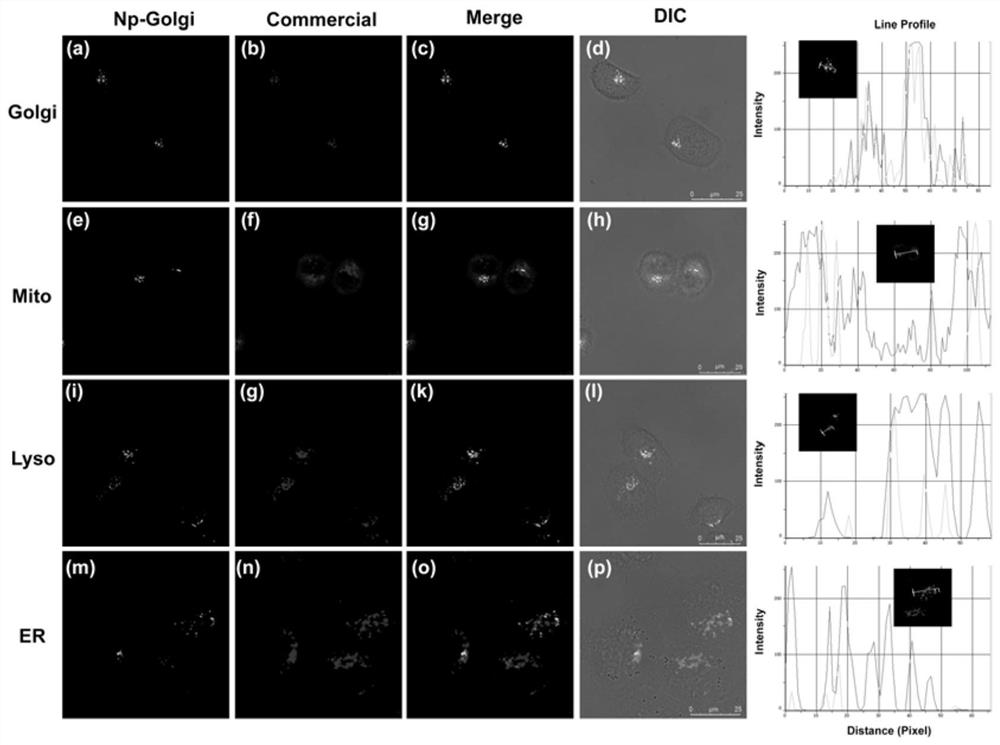
Compound of formula (i) based on benzenesulfonamide structure and its preparation method and application
A technology of compound and structural formula, applied in the field of compound of formula (I) based on benzenesulfonamide structure and its preparation and application, to achieve the effect of less damage to cells and living organisms, simple design strategy and synthetic route, and high response multiple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Synthesis of fluorescent probes
[0042] Dissolve the raw material 4-bromo-1,8-naphthalic anhydride (0.3g) and bis-pinacol borate (0.38g) in 15mL of 1,4-dioxane, and then add PdCl 2 (dppf)(0.0732g) and K 2 CO3 (0.3g), reflux at 90°C for 12h. After the reaction was complete, the solvent was removed by rotary evaporation. Subsequently, the compound was purified by column chromatography using dichloromethane:methanol=20:1 as the eluent to obtain the white solid intermediate product Np-Cyto (62%).
[0043] The intermediate product Np-Cyto (0.3g) and sulfonamide (1.7g) were dissolved in 15mL of acetic acid, and heated at reflux at 160°C for 12h. After the reaction was complete, the solvent was removed by rotary evaporation. Subsequently, the compound was purified by column chromatography with dichloromethane:methanol 10:1 to obtain light yellow powder Np-Golgi (10% yield).
[0044] NMR and mass spectrometry characterization:
[0045] 1 H NMR (400MHz, CDCl 3 )δ9.20(d,...
Embodiment 2
[0057] Synthesis of fluorescent probes
[0058] Dissolve the raw material 4-bromo-1,8-naphthalic anhydride (0.277g) and double pinacol borate (0.35g) in 15mL of 1,4-dioxane, then add PdCl 2 (dppf)(0.082g) and K 2 CO 3 (0.32g), reflux at 90°C for 10h. After the reaction was complete, the solvent was removed by rotary evaporation. Subsequently, the compound was purified by column chromatography using dichloromethane:methanol=20:1 as the eluent to obtain the white solid intermediate product Np-Cyto (42%).
[0059] Take the intermediate product Np-Cyto (0.325g) and sulfonamide (0.85g), dissolve it in 12mL of acetic acid, and heat at reflux at 140°C for 12h. After the reaction was complete, the solvent was removed by rotary evaporation. Subsequently, the compound was purified by column chromatography with dichloromethane:methanol 10:1 to obtain light yellow powder Np-Golgi (yield 6%).
Embodiment 3
[0061] Synthesis of fluorescent probes
[0062] Dissolve the raw material 4-bromo-1,8-naphthalic anhydride (0.28g) and bis-pinacol borate (0.40g) in 15mL of 1,4-dioxane, and then add PdCl 2 (dppf)(0.075g) and K 2 CO 3 (0.31g), reflux at 90°C for 12h. After the reaction was complete, the solvent was removed by rotary evaporation. Subsequently, the compound was purified by column chromatography using dichloromethane:methanol=20:1 as the eluent to obtain the white solid intermediate product Np-Cyto (52%).
[0063] The intermediate product Np-Cyto (0.33g) and sulfonamide (1.2g) were dissolved in 14mL of acetic acid and heated under reflux at 150°C for 12h. After the reaction was complete, the solvent was removed by rotary evaporation. Subsequently, the compound was purified by column chromatography with dichloromethane:methanol 10:1 to obtain light yellow powder Np-Golgi (yield 7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com