Krebs-type polyacid compound and preparation method thereof
A multi-acid compound and chemical formula technology, applied in the field of anti-tumor drugs, can solve the problems of high toxicity, tumor cell drug resistance, poor selectivity, etc., and achieve the effect of low toxicity, strong cell penetration ability, and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The {SbW adopted in the following examples 9} preparation reference [M. I. Loose, H. Pohlmann,
[0036] B. Krebs. Chem. Eur. J. 8 (1997) 1232-1233].
Embodiment 1
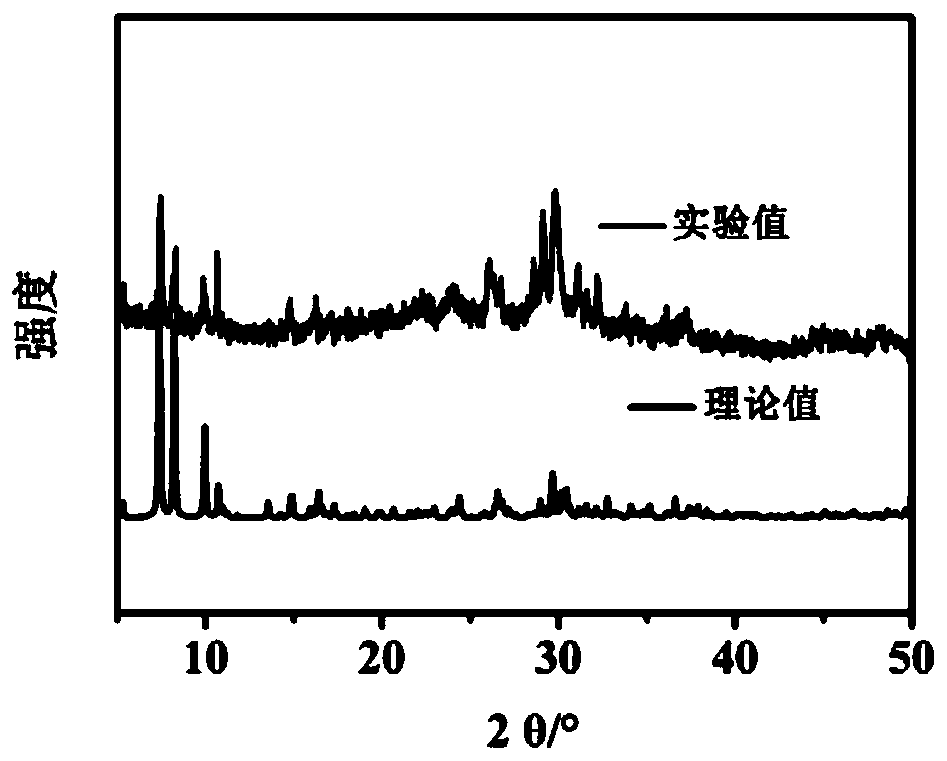
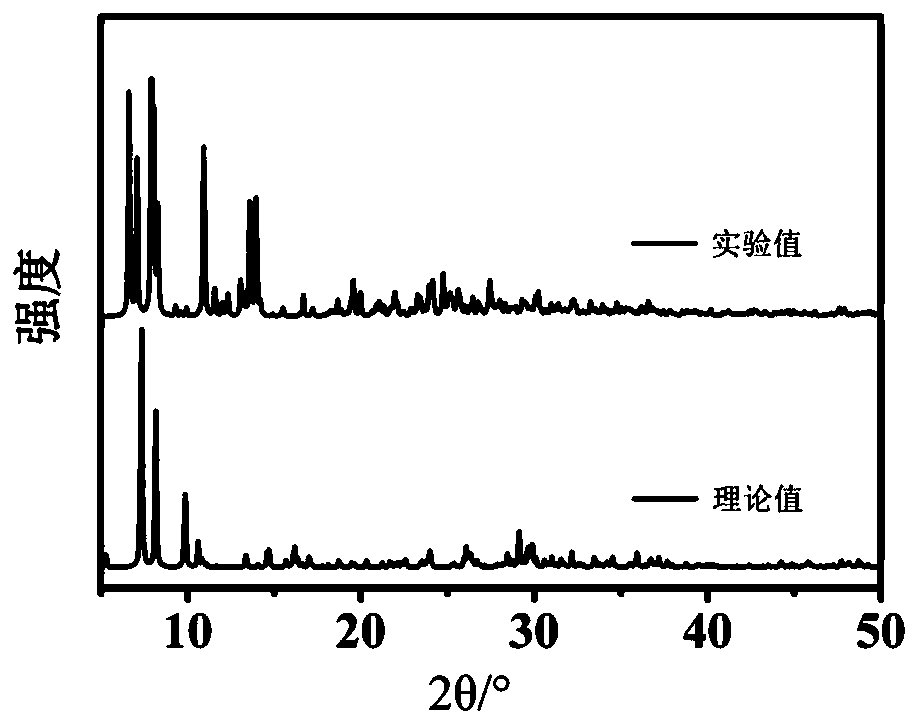
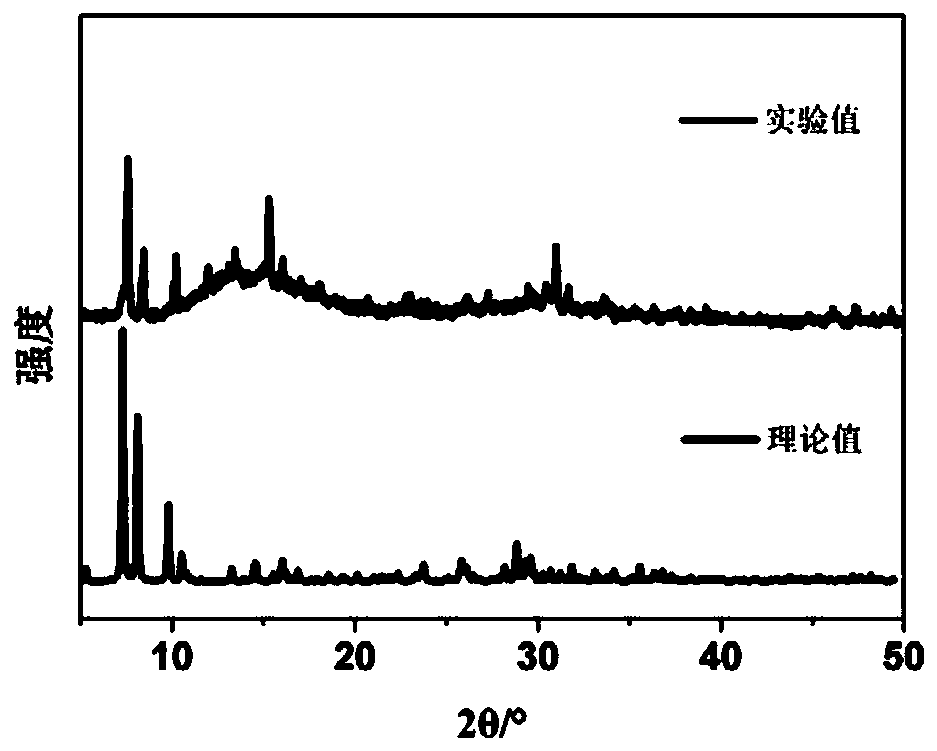
[0039] Weigh 110mg{SbW 9} was dissolved in 30mL of distilled water, stirred to dissolve, and then 90mg of Mn(CH 3 COO) 2 4H 2 O, after stirring and dissolving, add 35 mg of 1,2,4-triazole. After all the substances are dissolved, adjust the pH of the solution to 3.0 with acetic acid, and the color of the solution changes from orange to light yellow. Naturally volatilize at room temperature for 15 to 20 days, and crystallize to obtain a yellow block single crystal, that is, the Krebs type polyacid compound Na 2 mn 2 h 6 [Mn 2 (H 2 O) 2 (trz) 2 (SbO 2 ) 2 (SbW 9 o 33 ) 2 ](POM-Mn), its structure see Figure 9 . The yield was 43.25%, based on {SbW 9}.
Embodiment 2
[0041] Weigh 110mg{SbW 9} was dissolved in 30mL of distilled water, stirred to dissolve, and then 90mg of Mn(CH 3 COO) 2 4H 2 O Stir to dissolve and add 35 mg of 1,2,4-triazole. After all the substances are dissolved, adjust the pH of the solution to 2.0 with acetic acid, and the color of the solution changes from orange to light yellow. Naturally volatilize at room temperature for 15 to 20 days, and crystallize to obtain a yellow block single crystal, that is, the Krebs type polyacid compound Na 2 mn 2 h 6 [Mn 2 (H 2 O) 2 (trz) 2 (SbO 2 ) 2 (SbW 9 o 33 ) 2 ] (POM-Mn).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com