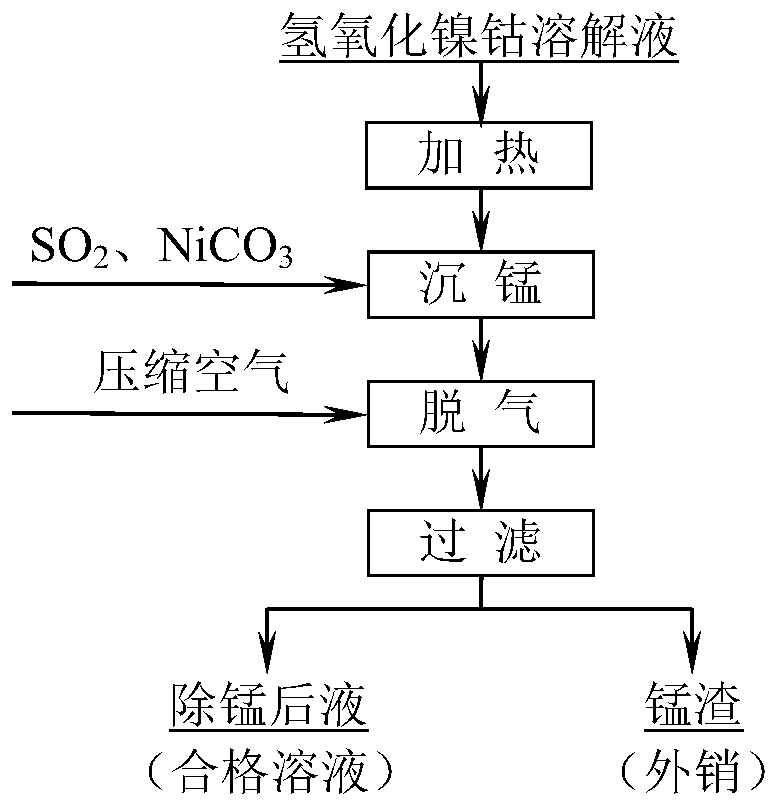
Method for removing manganese in nickel cobalt hydroxide solution
A nickel-cobalt hydroxide and solution technology, which is applied in the direction of improving process efficiency, can solve the problems of low cost, high manganese content, and difficult removal, and achieve the effects of increasing temperature, increasing reaction speed, and reducing metal entrainment of manganese slag Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Table 1 example 1 used nickel hydroxide cobalt solution composition (g / L)
[0025] Ni mn co Fe 180.12 5.44 5.23 0.14
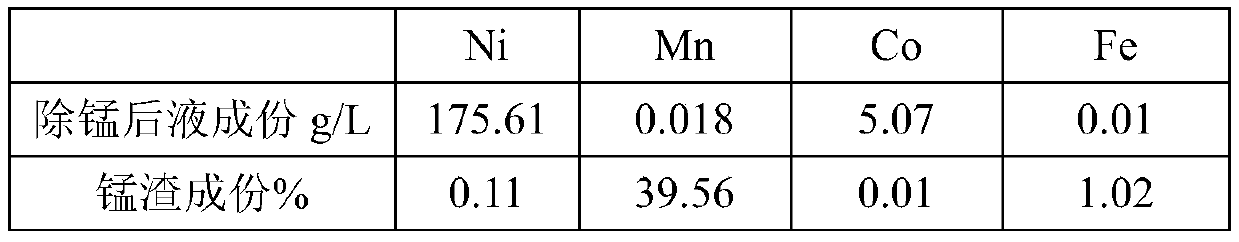
[0026] The specific implementation process: take a certain amount of nickel hydroxide cobalt hydrochloric acid solution, after heating up to 90 ° C, slowly and evenly pass into industrial sulfur dioxide gas (volume concentration 30%) of 1.3 times the theoretical amount, and the reaction time of ventilation is 4h. Nickel controls the pH of the reaction slurry to be 3.0; after the reaction is completed, inject compressed air into the feed liquid for 10 minutes; filter to obtain the manganese-precipitated liquid and manganese-precipitated slag. Table 2 shows the manganese removal liquid, manganese slag components and slag-calculated manganese removal rate.
[0027] Table 2 manganese removal example 1 analysis result
[0028]
[0029]
Embodiment 2
[0031] The used nickel hydroxide cobalt solution composition of table 3 example 2 (g / L)
[0032] Ni mn co Fe 187.27 6.33 5.72 0.23
[0033] The specific implementation process: take a certain amount of nickel hydroxide cobalt hydrochloric acid solution, after heating up to 105 ° C, slowly and evenly pass into industrial sulfur dioxide gas (volume concentration 30%) of 1.4 times the theoretical amount, and the reaction time of ventilation is 5h. Nickel controls the pH of the reaction slurry to be 3.5; after the reaction is completed, inject compressed air into the feed liquid for 12 minutes; filter to obtain the manganese-precipitated liquid and manganese-precipitated slag. Table 4 shows the manganese removal liquid, manganese slag components and slag-calculated manganese removal rate after manganese removal.
[0034] Table 4 manganese removal example 2 analytical results
[0035]
Embodiment 3
[0037] Table 5 example 3 used nickel hydroxide cobalt solution composition (g / L)
[0038] Ni mn co Fe 198.4 6.84 6.95 0.47
[0039] The specific implementation process: take a certain amount of nickel hydroxide cobalt hydrochloric acid solution, after heating up to 110 ° C, slowly and evenly pass into industrial sulfur dioxide gas (volume concentration 30%) of 1.5 times the theoretical amount, and the reaction time of ventilation is 6h. Nickel controls the pH of the reaction slurry to be 4.0; after the reaction is completed, inject compressed air into the feed liquid for 15 minutes; filter to obtain the manganese-precipitated liquid and manganese-precipitated slag. Table 6 shows the manganese removal liquid, manganese slag composition and slag manganese removal rate after manganese removal.
[0040] Table 6 manganese removal example 3 analytical results
[0041]
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com