Short-acting heparin-based anticoagulant compounds and methods
An anticoagulant activity, heparin technology, applied in chemical instruments and methods, separation methods, chemical/physical processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
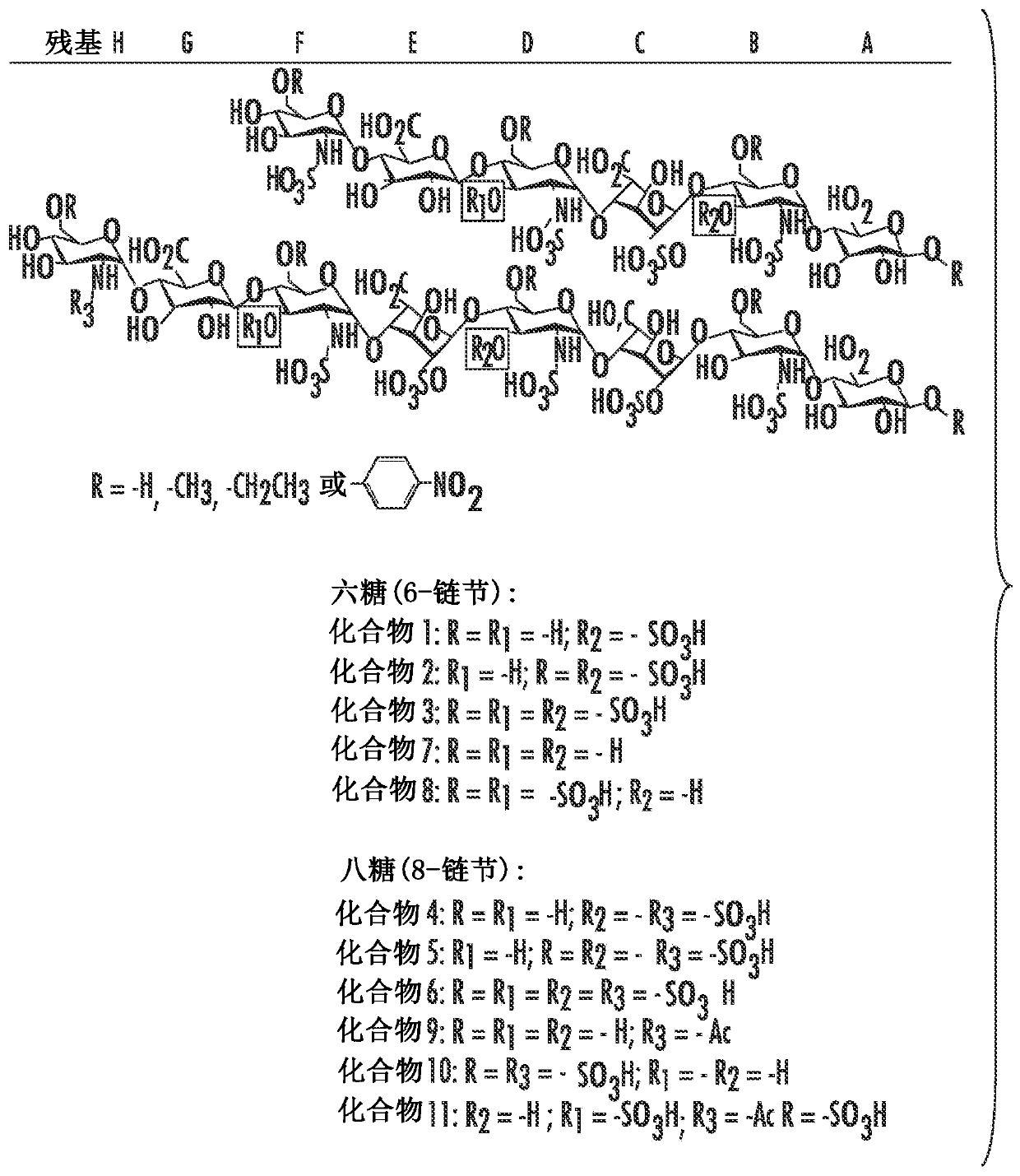
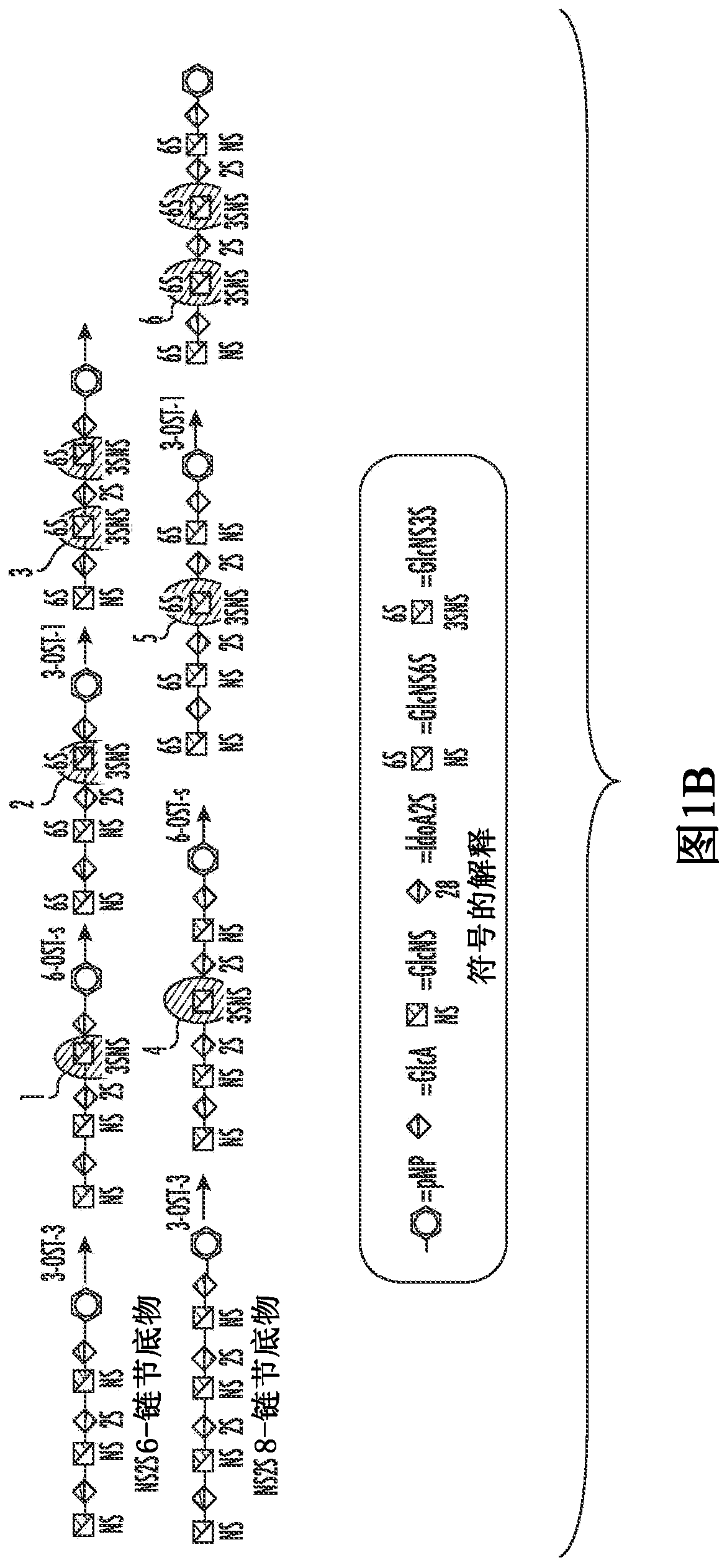
[0045]Synthetic methods for the synthesis of heparin analogs are shown (see, e.g., Figure 1A , Figure 1B and Figure 8C ) and are discussed further herein, but can include, in certain aspects, providing a sugar substrate, extending the sugar substrate to a sugar of a desired or predetermined length, using a 3-O-sulfotransferase ( The 3-OST-3 isoform of the 3-OST) enzyme undergoes at least one sulfation reaction whereby a synthetic heparin analog is synthesized. The sugar substrate may comprise at least one IdoA2S-GlcNS3S disaccharide unit.
[0046] The disclosed synthetic methods for the synthesis of heparin analogs provide surprisingly high yields of heparin compounds. By way of example and not limitation, the disclosed synthetic methods for the synthesis of heparin analogs can have greater than about 20%, greater than about 30%, greater than about 40%, greater than about 50%, about 20% to about 50%, about 30% to About 50%, or about 40% to about 50% yield.
[0047] Whe...
Embodiment 1
[0146] Chemoenzymatic Synthesis of Oligosaccharides Carrying GlcNS3S and GlcNS3S6S Residues
[0147] In this study, hexasaccharides (compounds 1-3, Figure 1A ) and octasaccharides (compounds 4-6, Figure 1A )Synthesis. Two 3-O-sulfotransferase (3-OST) isoforms (3-OST-1 and 3-OST-3) were used to install GlcNS3S±6S residues into different sugar sequences. The 3-OST-1 enzyme introduces sulfation to form a GlcNS3S6S residue, which is linked to a GlcA residue at the non-reducing end to form a disaccharide unit of -GlcA-GlcNS3S6S-; and the 3-OST-3 enzyme Sulfation is introduced to form a GlcNS3S residue, which is linked at the non-reducing end to an IdoA2S residue, forming a disaccharide unit of -IdoA2S-GlcNS3S-. Although 3-OST-1 has been successfully used in the synthesis of oligosaccharides in many studies 16,17,27,28 , but using 3-OST-3 to synthesize oligosaccharides comprising the -IdoA2S-GlcNS3S- disaccharide unit has not been reported yet.
[0148] Disclosed herein is th...
Embodiment 2
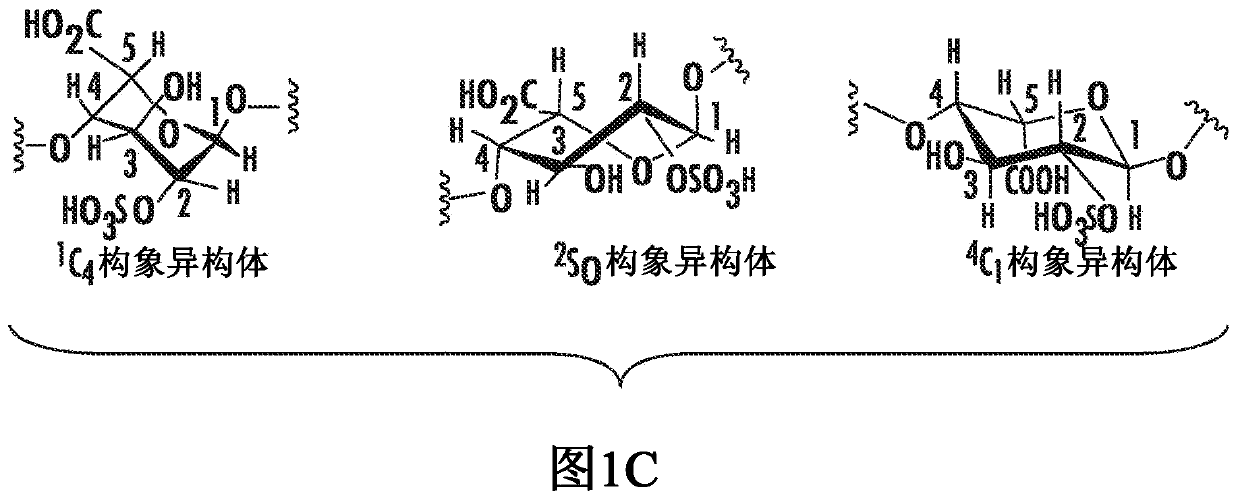
[0154] Structural and conformational analysis of oligosaccharides
[0155] Purity and structure analysis of compounds 1 to 6 were carried out. Figure 6E Representative data for compound 6 shown in Figure 2A and Figure 2B shown in . Compound 6 eluted as a single peak by high-resolution anion-exchange HPLC, indicating that the compound was pure ( Figure 2A ). The molecular weight of compound 6 measured by electrospray ionization mass spectrometry (ESI-MS) is 2449.43 ± 0.74, which is very close to the calculated molecular weight of 2448.92 ( Figure 2B ). The 1H-NMR spectrum of compound 6 clearly shows 8 anomeric protons, confirming that the product is an octasaccharide. The 13C-NMR and full NMR assignments of compound 6 are shown in Supplementary Figure 25 and Supplementary Table 1, respectively. To locate the 3-O-sulfo group in compound 4, tandem MS analysis was performed. In this assay, the introduction of a stable isotopically labeled [34S]sulfo group by the 3-OST...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Binding affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com