Cryopreservation solution, cryopreservation method and recovery method for peripheral blood mononuclear cells
A cryopreservation method and nuclear cell technology, which is applied in the field of cryopreservation solution for peripheral blood mononuclear cells, can solve the problems of unknown cell viability and reduced cell viability, and achieve the effect of high viability and high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Freezing solution 1
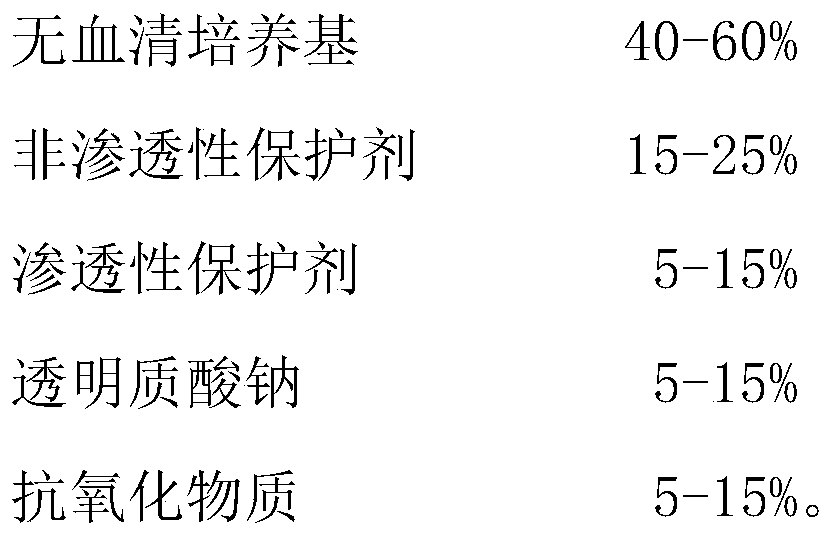
[0024] The cryopreservation solution consists of the following components: 500 μL X-VIVO15 serum-free medium, 200 μL human albumin, 100 μL 0.5 wt% sodium hyaluronate, 100 μL 50 μg / mL ascorbic acid, and 100 μL DMSO.
[0025] In other alternative embodiments, the cryopreservation solution can be composed of the following components: 400 μL X-VIVO15 serum-free medium, 150 μL human albumin, 150 μL 0.5wt% sodium hyaluronate, 150 μL 50 μg / mL ascorbic acid, and 150 μL DMSO ; It can also be composed of the following components: 600 μL X-VIVO15 serum-free medium, 250 μL human albumin, 50 μL 0.5wt% sodium hyaluronate, 50 μL 50 μg / mL ascorbic acid, 50 μL DMSO.
Embodiment 2
[0036] Embodiment 2PBMC separation
[0037] The peripheral blood was collected by anticoagulation, and the upper plasma after centrifugation was set aside. According to the ratio of 1:1, the plasma-removed components in the centrifuge tube were gradually added to the upper layer of the lymphocyte separation medium along the tube wall, and the buffy coat cells were collected after centrifugation, washed with X-VIVO15 serum-free medium for 3 times, and re- Hang count.
Embodiment 3PB
[0038] Embodiment 3 PBMC cryopreservation
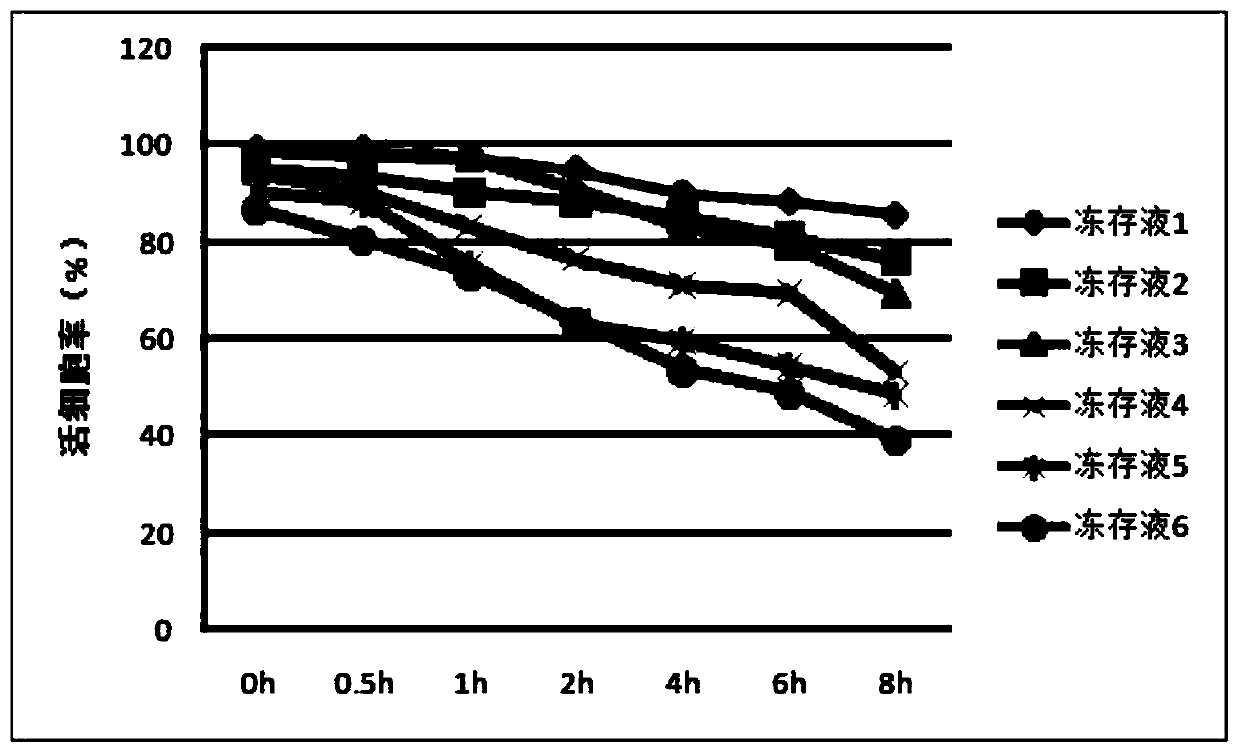
[0039] Transfer the counted cell suspension into 15ml centrifuge tubes, discard the supernatant after centrifugation, transfer to 6 cryopreservation tubes and add cryopreservation solutions 1-6, each cryopreservation tube freezes 1.87*10 7 cells, cryopreservation volume 1mL. Seal the cryopreservation tube, place it in a cooling box, and quickly transfer it to a -80°C refrigerator. After overnight, transfer the cryopreservation tube to a liquid nitrogen tank at -196°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com