Test strip for simultaneously and quantitatively detecting NSE and S100B in blood and preparation method thereof
A quantitative detection and test strip technology, which is applied in the field of medical detection, can solve the problems of inability to realize ultra-early diagnosis, high professional level requirements, and expensive detection instruments, so as to achieve good clinical application significance, improve accuracy and sensitivity, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Example 1, this example provides a magnetic immunochromatographic test strip and a preparation method for quantitatively detecting NSE and S100B in blood at the same time.
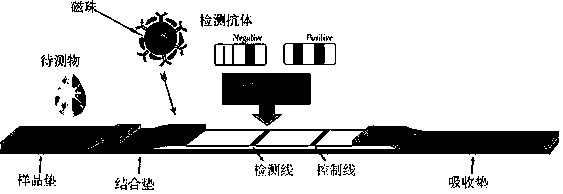
[0030] Both the NSE paired antibody and the S100B paired antibody used in this example are monoclonal antibodies prepared by monoclonal antibody technology. The principle of double-antibody sandwich detection of NSE and S100B antigens is used to detect specimens. When the specimen to be tested contains NSE and S100 antigens, the antigens will first bind to the magnetic beads coupled with NSE antibody and the magnetic beads coupled with S100B antibody on the binding pad. , as the chromatography progresses, the conjugate moves forward to reach the S100B antibody detection line T2, the S100B antigen will combine with the coating antibody again to form a double antibody sandwich complex and gather at T2, and the conjugate moves forward to reach the NSE antibody At the detection line T1, the NSE antigen w...
Embodiment 2
[0053] Except in the preparation step of immunomagnetic beads: the addition amount of EDC and NHS is 5 times the molar amount of carboxylation degree of magnetic beads, other steps are the same as in Example 1.
Embodiment 3
[0055] Except that in the preparation step of the conjugate pad, the content of sucrose in the conjugate pad treatment solution used was 10 wt%, other steps were the same as in Example 1.
[0056] The use method of detection card of the present invention
[0057] 1. Add sample
[0058] Take out the test card for a single person from the packaging box, tear open the aluminum foil packaging bag, place it on a flat table, use a micropipette to pipette 70 μL of serum sample into the sample hole of the test card, and wait for the reaction to proceed for 20 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com