Low-concentration retapamulin determination method and application
A technology of retamoline and determination method, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., to achieve the effects of high sensitivity and accuracy, strong specificity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 Determination of retapamulin ointment transdermal test receiving liquid sample
[0058] Chromatographic conditions:
[0059] Chromatographic column: SHIMADZUVP-C18250×4.6mm, 5μm;
[0060] Mobile phase: methanol: 10mM ammonium acetate aqueous solution (75:25);
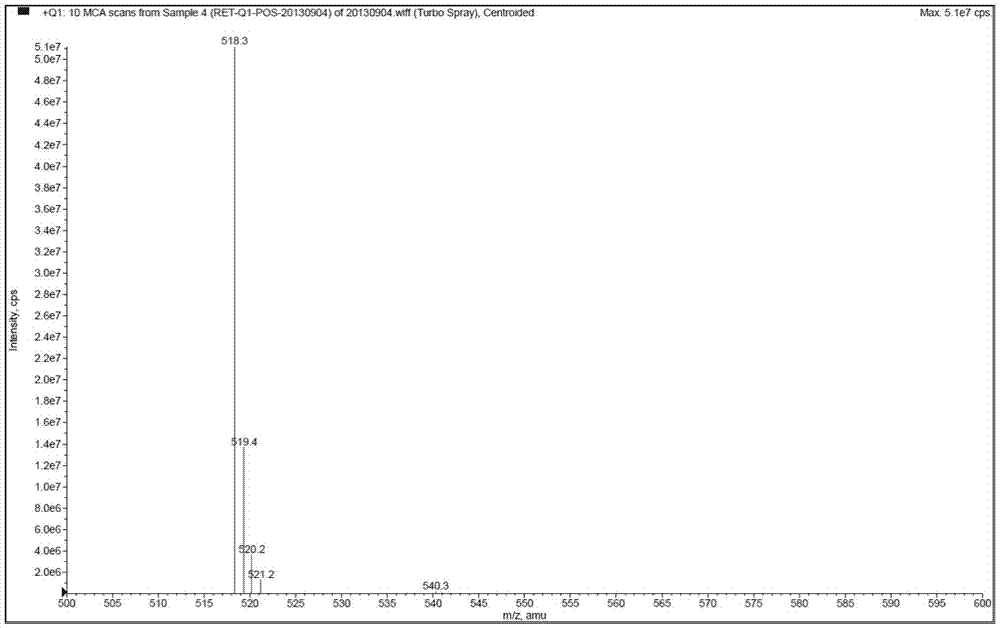
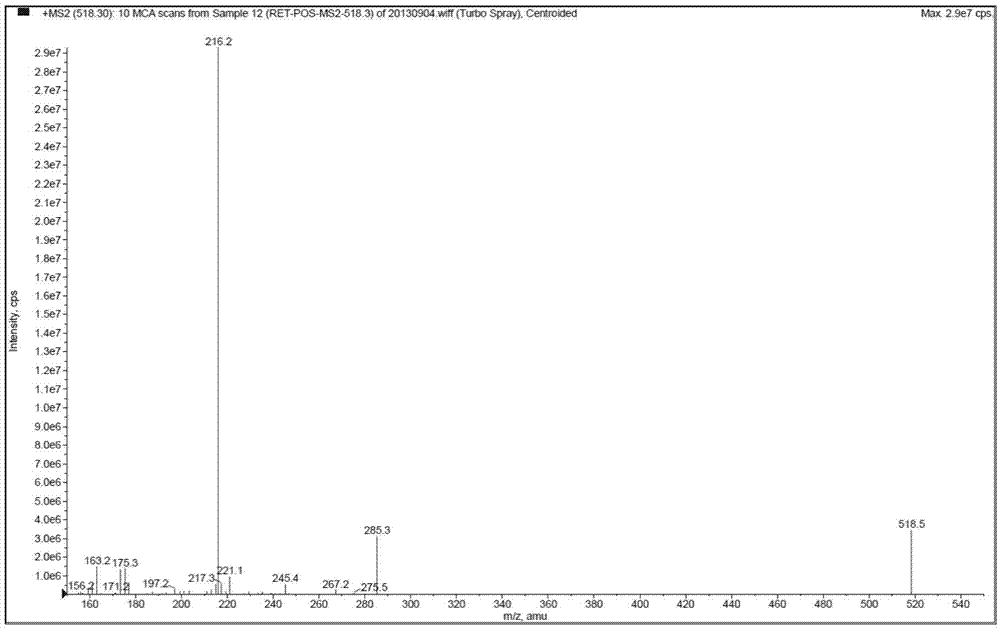
[0061] Mass spectrometry conditions: detection in positive ion mode; drying gas: N 2 ;Drying gas pressure (Gas2): 70psi; Ionization temperature (TEM) is 300°C; Atomization gas (Gas1) is 9psi; Curtain Gas (CurtainGas) is 12psi; Spray voltage (IonSprayVoltage) is 4700V; Collision gas (CAD) 6psi; injection voltage (EP) 10V; collision cell exit voltage (CXP) 20V;
[0062]
[0063] Detection method:
[0064] Standard curve and quality control: take retapamulin reference substance solution and tiamulin reference substance solution, respectively prepare 0.504ng / ml, 1.01ng / ml, 2.02ng / ml, 5.04ng / ml, 20.2ng / ml A series of solutions of , 40.3ng / ml and 60.5ng / ml are used as the standard curve, and the pe...
Embodiment 2
[0066] The determination of the intradermal sample of embodiment 2 retapamulin ointment transdermal test
[0067] Chromatographic conditions:
[0068] Chromatographic column: SHIMADZUVP-C18250×4.6mm, 5μm;
[0069] Mobile phase: methanol: 10mM ammonium acetate aqueous solution (75:25);
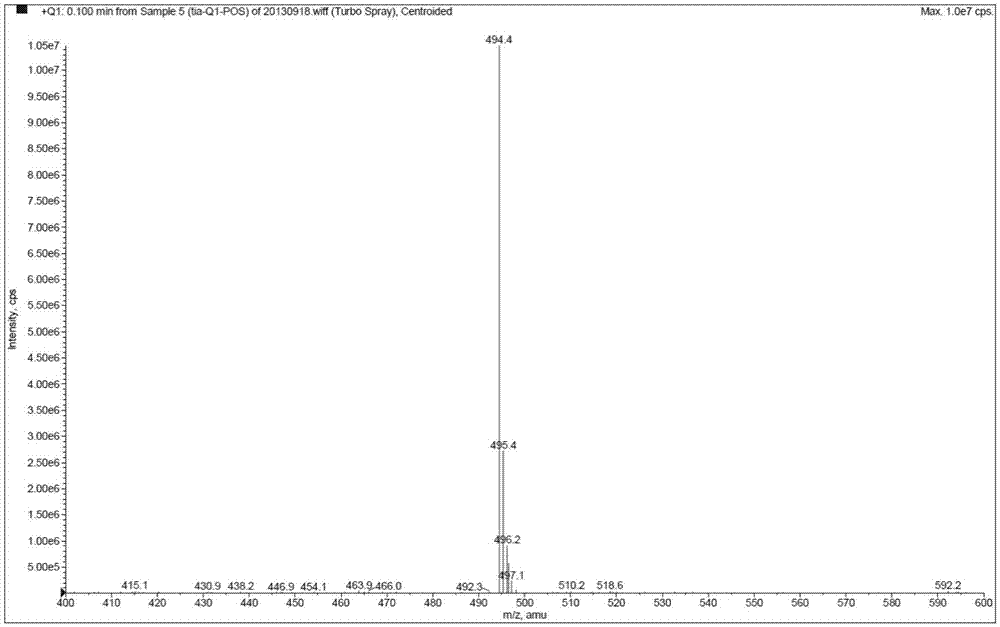
[0070] Mass spectrometry conditions: detection in positive ion mode; drying gas: N 2 ;Drying gas pressure (Gas2): 70psi; Ionization temperature (TEM) is 300°C; Atomization gas (Gas1) is 9psi; Curtain Gas (CurtainGas) is 12psi; Spray voltage (IonSprayVoltage) is 4700V; Collision gas (CAD) 6psi; injection voltage (EP) 10V; collision cell exit voltage (CXP) 20V;
[0071]
[0072] Detection method:
[0073] Standard curve and quality control: take retapamulin reference substance solution and tiamulin reference substance solution, respectively prepare a series of solutions of 101ng / ml, 126ng / ml, 151ng / ml, 176ng / ml and 202ng / ml as For the standard curve, the peak area is determined by injecti...
Embodiment 3
[0075] The determination of the sample on the skin of embodiment 3 retapamulin ointment transdermal test
[0076] Chromatographic conditions:
[0077] Chromatographic column: SHIMADZUVP-C18250×4.6mm, 5μm;
[0078] Mobile phase: methanol: 10mM ammonium acetate aqueous solution (75:25);
[0079] Mass spectrometry conditions: detection in positive ion mode; drying gas: N 2 ;Drying gas pressure (Gas2): 70psi; Ionization temperature (TEM) is 300°C; Atomization gas (Gas1) is 9psi; Curtain Gas (CurtainGas) is 12psi; Spray voltage (IonSprayVoltage) is 4700V; Collision gas (CAD) 6psi; injection voltage (EP) 10V; collision cell exit voltage (CXP) 20V;
[0080]
[0081] Detection method:
[0082] Standard curve and quality control: take retapamulin reference substance solution and tiamulin reference substance solution, respectively prepare a series of solutions of 161ng / ml, 181ng / ml, 202ng / ml, 222ng / ml and 242ng / ml as For the standard curve, the peak area is determined by injecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com