Cefoperazone sodium for injection and preparation method thereof
A technology for cefoperazone sodium and injection, applied in the field of medicine, can solve problems such as poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
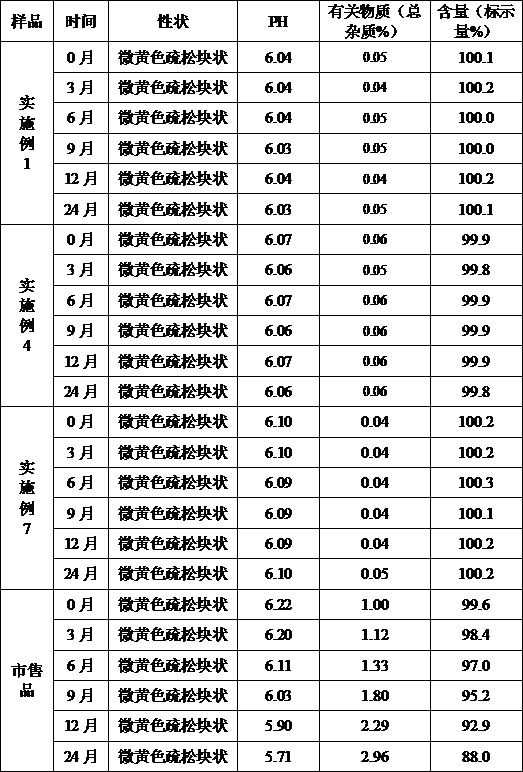
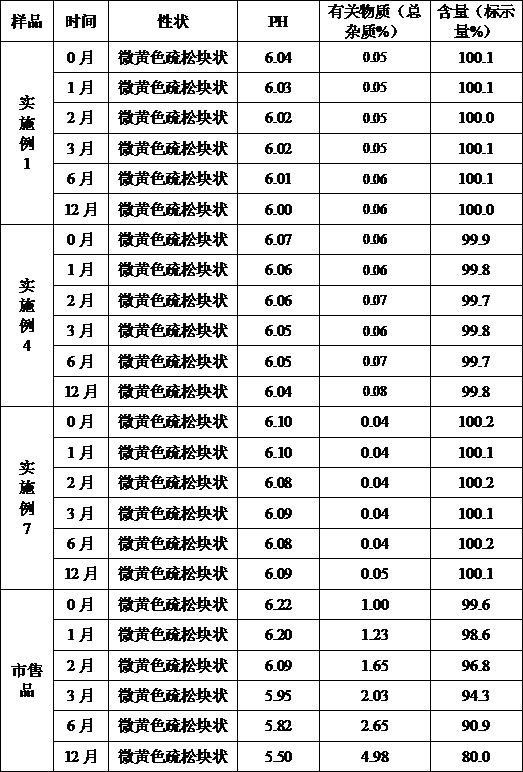
[0043] Example 1 Preparation of Cefoperazone Sodium for Injection (Specification: 0.5g)
[0044] prescription:
[0045] Cefoperazone Sodium: 500g
[0046] Sodium citrate: 12g
[0047] Povidone K30: 10g
[0048] Add water for injection to 2000ml
[0049] Make 1000 pieces
[0050] The preparation method of cefoperazone sodium for injection of the present invention comprises the following steps:
[0051] (1) Add the prescribed amount of sodium citrate, povidone K30, and cefoperazone sodium into 1200ml of water for injection in turn, stir and dissolve, add water for injection to 2000ml, and stir evenly;
[0052] (2) Filter the solution in step (1) through a 0.22 μm microporous membrane, measure the pH value and content of the filtrate, determine the filling volume according to the specifications, and divide into half-stoppered liquid;
[0053] (3) Freeze-drying:
[0054] ①Pre-freezing: put the aliquot in a freezer that has been cooled to -36°C in advance, and keep it for 3 ...
Embodiment 2
[0057] Example 2 Preparation of Cefoperazone Sodium for Injection (Specification: 0.5g)
[0058] prescription:
[0059] Cefoperazone Sodium: 500g
[0060] Sodium citrate: 12g
[0061] Povidone K30: 10g
[0062] Add water for injection to 2000ml
[0063] Made 1000 pieces
[0064] The preparation method of cefoperazone sodium for injection of the present invention comprises the following steps:
[0065] (1) Add the prescribed amount of sodium citrate, povidone K30, and cefoperazone sodium into 1200ml of water for injection in turn, stir and dissolve, add water for injection to 2000ml, and stir evenly;
[0066] (2) Filter the solution in step (1) through a 0.22 μm microporous membrane, measure the pH value and content of the filtrate, determine the filling volume according to the specifications, and divide into half-stoppered liquid;
[0067] (3) Freeze-drying:
[0068] ① Pre-freezing: place the aliquot in a freezer that has been cooled to -24°C in advance, and keep it for...
Embodiment 3
[0071]Example 3 Preparation of Cefoperazone Sodium for Injection (Specification: 0.5g)
[0072] prescription:
[0073] Cefoperazone Sodium: 500g
[0074] Sodium citrate: 12g
[0075] Povidone K30: 10g
[0076] Add water for injection to 2000ml
[0077] Made 1000 pieces
[0078] The preparation method of cefoperazone sodium for injection of the present invention comprises the following steps:
[0079] (1) Add the prescribed amount of sodium citrate, povidone K30, and cefoperazone sodium into 1200ml of water for injection in turn, stir and dissolve, add water for injection to 2000ml, and stir evenly;
[0080] (2) Filter the solution in step (1) through a 0.22 μm microporous membrane, measure the pH value and content of the filtrate, determine the filling volume according to the specifications, and divide into half-stoppered liquid;
[0081] (3) Freeze-drying:
[0082] ① Pre-freezing: put the aliquot in a freezer that has been cooled to -30°C in advance, and keep it for 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com