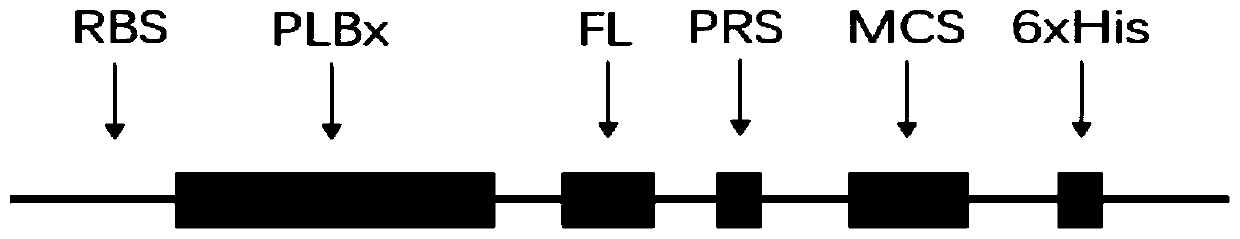
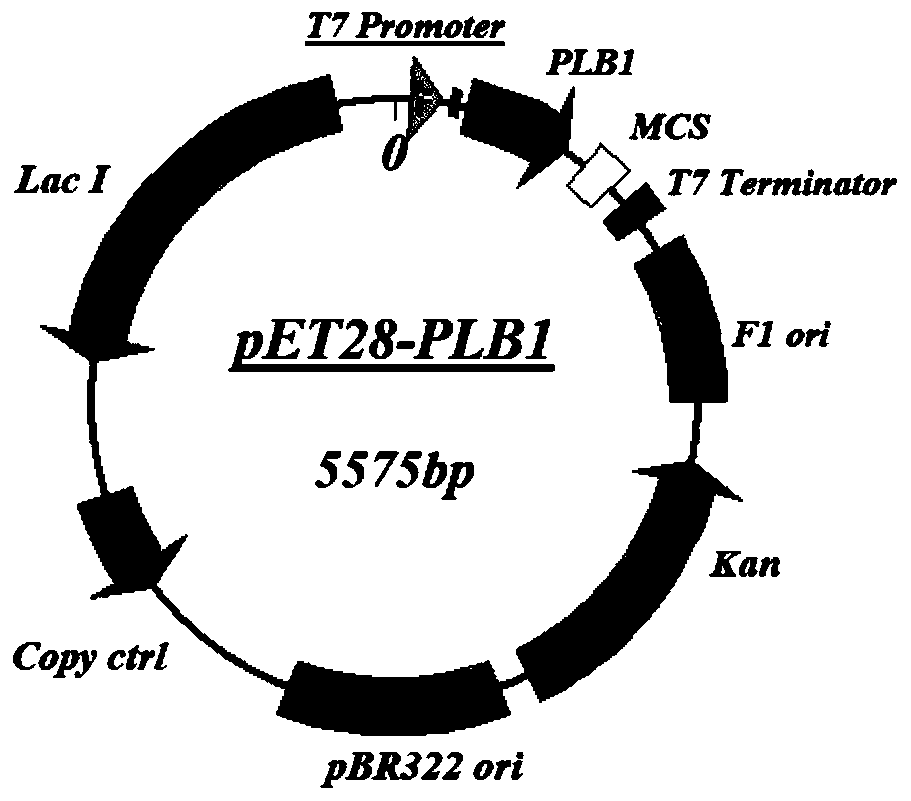
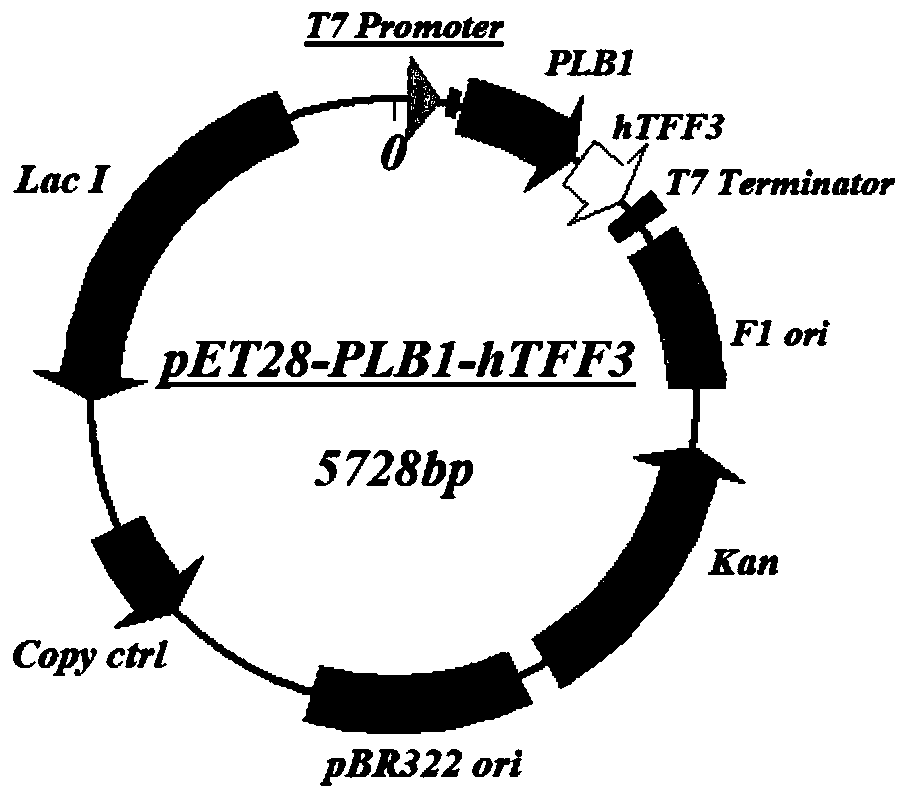
Application of PLB protein in construction of fusion protein expression vector with chaperonin-like protein effect
A technology for expressing vectors and fusion proteins, which is applied in the direction of introducing foreign genetic material, application, and fusion polypeptides by using vectors, and can solve the problems of limited expression vectors, differences, and unknown mechanisms of chaperone-like proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Construction of the Escherichia coli expression vector comprising chaperone-like protein
[0061] Step 1. Gene synthesis and vector preparation of the PLB insert:
[0062] Select PLB1 as shown in SEQ ID: 1, carry out codon optimization of PLB1 encoding DNA, addition mutation and DNA sequence optimization of transcription initiation region, selection of flexible linker and codon optimization, blood coagulation factor Xa (FXa) recognition sequence amino acid Codon optimization, the specific steps are as follows:
[0063] (1) The amino acid sequence of PLB1 is reverse-translated into a DNA coding sequence, and its own coding sequence (SEQ ID NO: 6, 7) is selected through codon preference.
[0064] (2) Select the high-expression translation initiation region peptide sequence (MASTYKLILNGKTS), optimize its coding sequence and insert it into the 5'-end of PLB1 coding DNA (SEQ ID NO: 6-9).
[0065] (3) A flexible linker (KEKTPEEQL) between the PLB1 coding region an...
Embodiment 2
[0086] Example 2 Construction of pET28-PLB1 fusion protein expression vector test system
[0087] Step 1. Using pET28-PLB1 as the parent vector and hTTF3 as the target protein to test, construct the pET28-PLB1-hTFF3 fusion protein expression vector and expression strain:
[0088] 1. Artificially synthesized DNA sequence encoding hTFF3 mature peptide (SEQ ID NO: 28), DNA codons were optimized by DNAWorks software (SEQ ID NO: 29), and 6 primers for whole gene synthesis (SEQ ID NO: 31-36), The PCR whole gene synthesis method is the same as in Example 1 (see step 1, section (8)).
[0089] 2. The DNA restriction endonuclease sites required for recombination are introduced into the PCR whole gene synthesis primers: EcoR I (5'-end primer, SEQ ID NO: 31) and Xho I (3'-end primer, SEQ ID NO: 36), which is convenient for double enzyme digestion and recombination connection;
[0090] 3. The PCR product synthesized by the hTFF3 gene and the pET28-PLB1 vector were respectively digested w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com