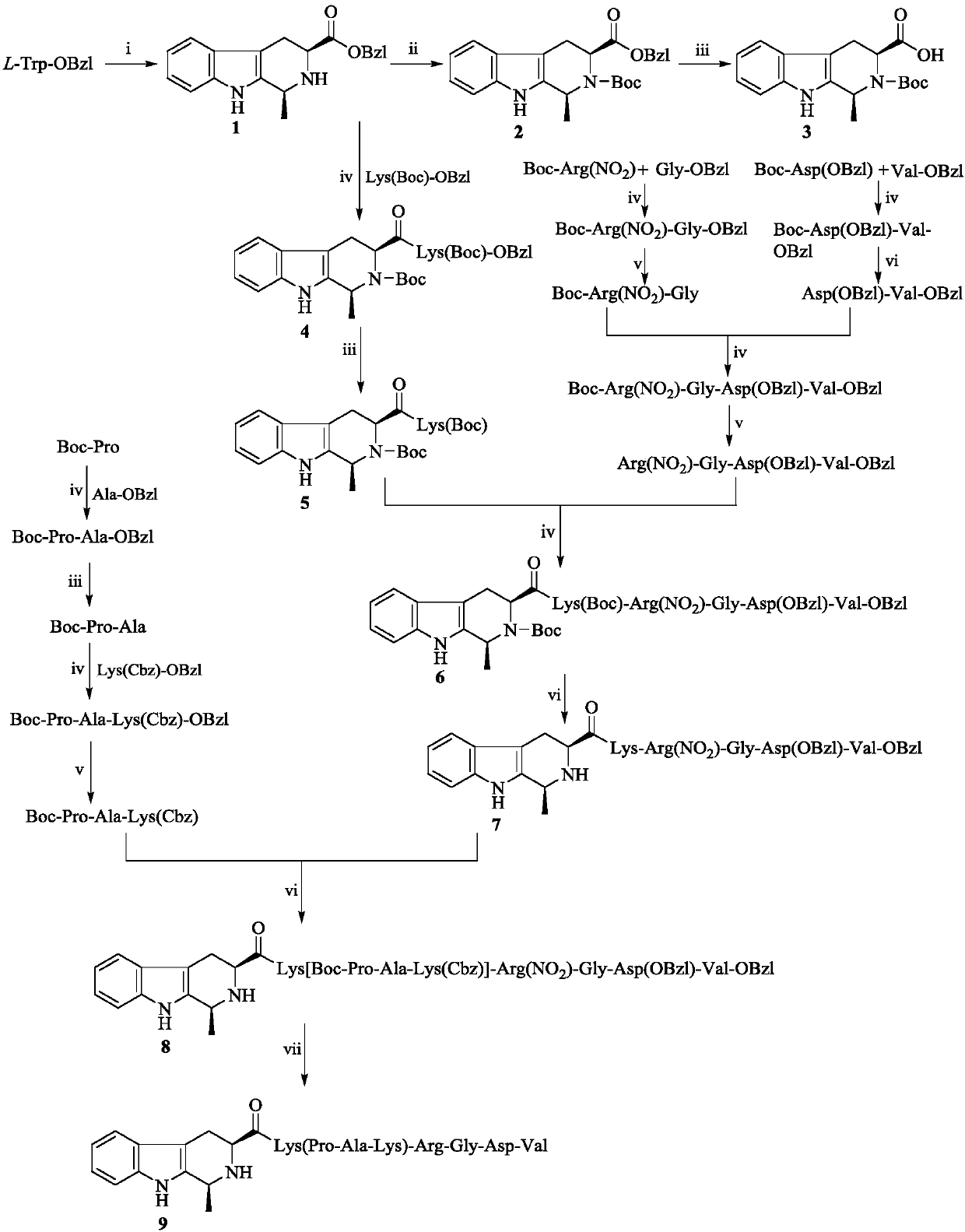
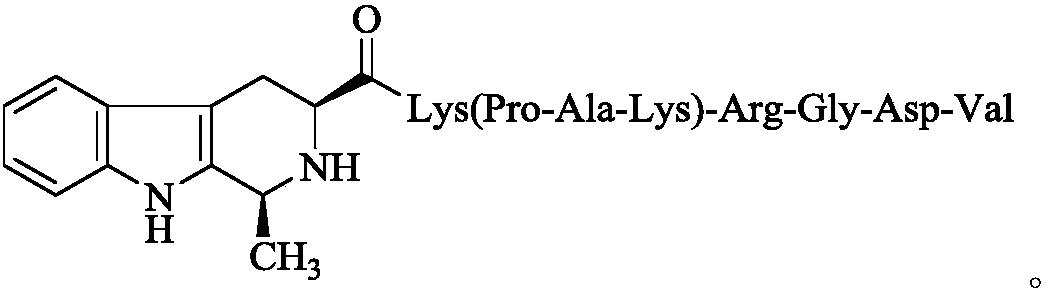1S-methyl-beta-tetrahydrocarbolineacyl-K(PAK)-RGDV, synthesis, activity and application thereof
A -arg-gly-asp-val, methyl technology, applied in 1S-methyl-β-tetrahydrocarboline-K (PAK)-RGDV, its synthesis, activity and application fields, can solve the preparation difficulty , Sensitive to the reducing environment, difficult to preserve, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0019] 1 mL of concentrated sulfuric acid with a concentration of 98% was added dropwise to 800 mL of distilled water, and after stirring evenly, 10.0 g (34 mmol) of L-Trp-OBzl was added therein three times. Stir for five minutes to fully suspend L-Trp-OBzl and sulfuric acid aqueous solution. Afterwards, 10 mL of 40% acetaldehyde aqueous solution was added dropwise to the suspension. The reaction mixture was stirred for 12 h, and then 3 mL of concentrated ammonia water was added dropwise to adjust the pH value of the reaction solution to 8. The reaction compound was allowed to stand for 1 h until the product was fully separated. The solid was filtered off and dried to yield 9.84 g (90%) of a pale yellow solid as 1R-methyl-1,2,3,4-tetrahydro-β-carboline-3S-carboxylate benzyl ester and 1S-methyl - Mixtures of benzyl 1,2,3,4-tetrahydro-β-carboline-3S-carboxylates. ESI-...
Embodiment 2
[0020] Example 2 Preparation of N-Boc-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (2)
[0021] 9.84g (30.8mmol) of 1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid benzyl ester obtained in Example 1 was mixed with 20mL N,N-dimethylformamide (DMF) dissolved. Add 6.98g (32.0mmol) (Boc) to the solution at 0°C 2 O. The resulting solution was adjusted to pH 12 with triethylamine and stirred at room temperature for 48 h. The reaction mixture was concentrated under reduced pressure to remove DMF. The residue was dissolved with 100 mL of ethyl acetate. The obtained ethyl acetate solution was washed successively with 5% potassium hydrogensulfate aqueous solution (50 mL×3) and saturated sodium chloride aqueous solution (50 mL×3). The separated ethyl acetate layer was dried over anhydrous sodium sulfate for 12 h, filtered, and the filtrate was concentrated under reduced pressure to obtain an oil. The oil was separated on a silica gel column (dich...
Embodiment 3
[0022] Example 3 Preparation of Boc-1S-methyl-1,2,3,4-tetrahydro-β-carboline-3S-carboxylic acid (3)
[0023] Add 100mg Pd / C, Stir to make a homogeneous suspension. The air in the reaction system was extracted under reduced pressure, hydrogen gas was introduced, and stirred at room temperature for 10 h. TLC (dichloromethane / methanol, 40 / 1) showed that N-Boc-1S-methyl-1,2,3,4-tetrahydro- β-carboline-3S-carboxylate benzyl ester completely disappeared. The Pd / C was removed by filtration, the filtrate was concentrated under reduced pressure, and the ESI-MS (m / e) of the obtained colorless powder was 329 [M-H] - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com