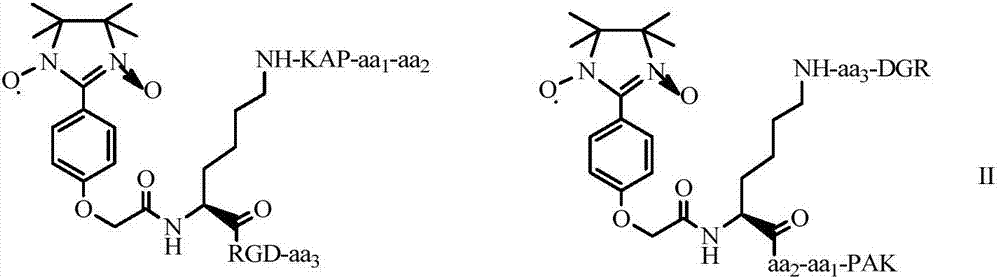
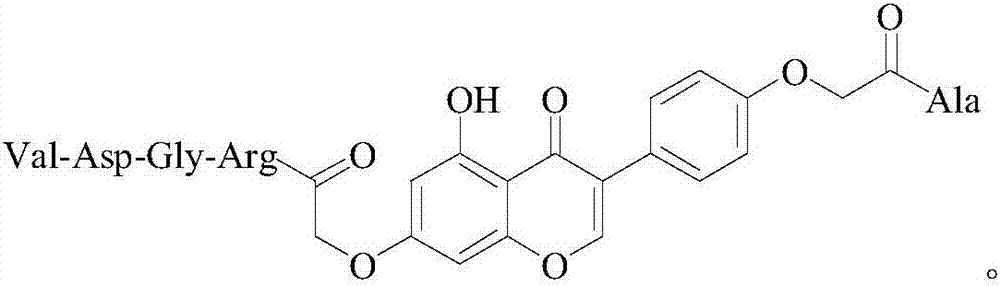
4'-oxyacetyl-Ala-5-hydroxyl-7-oxyacetyl-RGDV-isoflavone, and synthesis, activity and application thereof
A technology of hydroxyisoflavone and oxyacetyl, which is applied in the field of treatment of cerebral thrombosis, can solve the problems of inability to antagonize rat aortic strip relaxation, unsatisfactory, untreated ischemic stroke, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
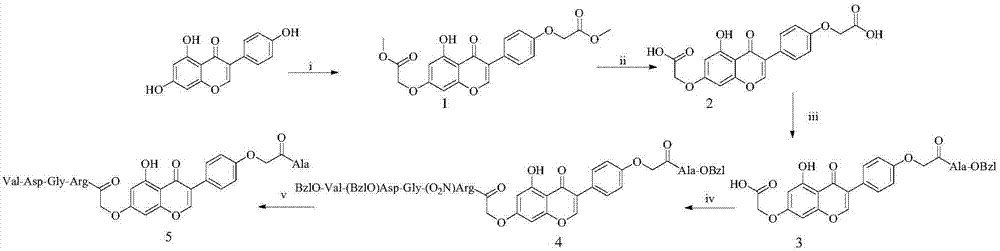
[0019] Example 1 Preparation of 4', 7-methyl dioxyacetate-5-hydroxy isoflavone (1)
[0020] Dissolve 11.69g (43.3mmol) of genistein in 200mL of tetrahydrofuran (THF), add 46.92g (340mmol) of potassium carbonate to the reaction solution, activate it for 20 minutes, add 14mL (87mmol) of ethyl bromoacetate, and place in a 45°C oil bath Under heating reaction for 5 days. The progress of the reaction was monitored by TLC (dichloromethane:methanol=20:1). After the reaction is complete, the reaction liquid is settled and filtered, the filtrate is decompressed to remove THF, and a large amount of petroleum ether is added to grind and wash to remove excess ethyl bromoacetate. The obtained solid is dissolved by adding methanol and found to be slightly soluble. After hot filtration, the solid was air-dried, and the filtrate was recrystallized by cold and heat, and the white solid was collected by filtration to obtain 11.70 g (61.1%) of the title compound. ESI - -MS(m / e):413[M-H] - . ...
Embodiment 2
[0021] Example 2 Preparation of 4', 7-dioxyacetic acid-5-hydroxy isoflavone (2)
[0022] Take 5.17g (12.5mmol) of methyl 4',7-dioxyacetate-5-hydroxyisoflavone (1) and add 100mL of methanol to make it slightly soluble. Add 2N NaOH aqueous solution dropwise to the reaction solution to adjust the pH value of the solution to 12. The solid gradually dissolved, and after reacting at room temperature for 4 hours, TLC (dichloromethane:methanol:glacial acetic acid=20:1:2 drops) monitored the reaction progress, and the raw material point disappeared, and the pH value of the solution was adjusted to 1-2 by adding saturated potassium bisulfate solution. A large amount of white solid was precipitated, and a large amount of water was added to dissolve the salt, and then filtered, and the filter cake was washed with water repeatedly, and the white solid was collected by drying to obtain 4.10 g (84.2%) of the title compound. ESI - -MS(m / e):385[M-H] - .
Embodiment 3
[0023] Embodiment 3 prepares 4'-oxyacetyl-Ala-OBzl-5-hydroxyl-7-oxyacetic acid-isoflavones (3)
[0024] Dissolve 1.59g (4.1mmol) of 4',7-dioxyacetic acid-5-hydroxyisoflavone (2) in 80mL of anhydrous N,N-dimethylformamide (DMF), and add to the reaction solution under ice-cooling Add 100mg (8.2mmol) 4-dimethylaminopyridine (DMAP) and 1.02g (4.9mmol) dicyclohexylcarbodiimide (DCC), activate for 20 minutes to obtain solution a; take 1.06g (4.9mmol) HCl A -OBzl was dissolved with anhydrous DMF and the pH value was adjusted to 8, the obtained solution was added to solution a, and the pH value of the reaction solution was adjusted to 8-9 using NMM, after 18 hours of reaction, the DMF was dried, and the obtained residue It was dissolved in dichloromethane, filtered, and the filtrate was separated and purified by column chromatography (dichloromethane / methanol) to obtain 1.50 g (66.8%) of the title compound. ESI - -MS(m / e):546[M-H] - .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com