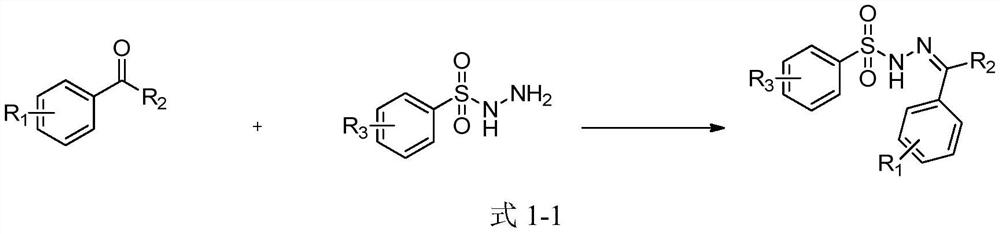
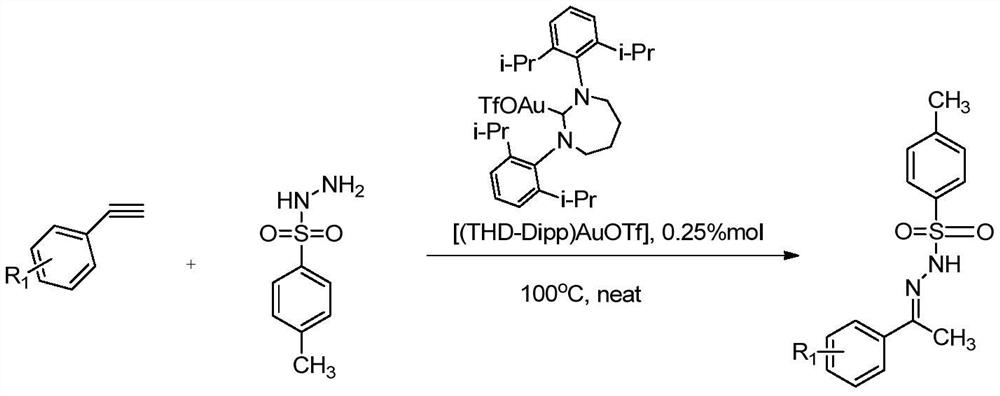
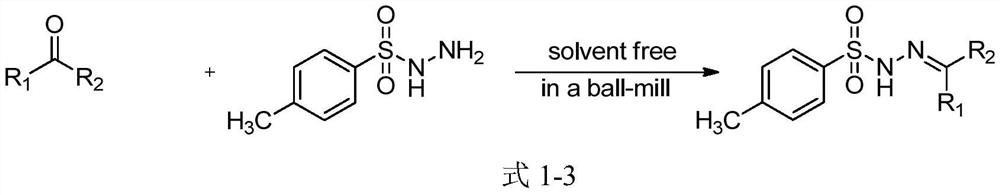
A kind of sulfonyl hydrazone derivative and its preparation method and application
A technology of derivatives and sulfonyl hydrazones, applied in the field of sulfonyl hydrazone derivatives and their preparation, can solve the problems of high yield and no target product, and achieve the effects of reducing pollution and eliminating pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~11
[0042] The solvent of embodiment 1~11 adopts CH 3 CN was reacted with different catalysts, and the reaction results are shown in Table 1.
[0043] Table 1 Reaction conditions and results of Examples 1-11
[0044]
[0045]
Embodiment 12~17
[0047] In Examples 12-17, no catalyst was added, and the reaction solvent was changed. The results of the reaction at 60° C. are shown in Table 2.
[0048] Table 2 Reaction conditions and results of Examples 12-17
[0049]
[0050] In Examples 18-20, no catalyst was added, water was used as the reaction solvent, and the results of changing the reaction temperature were shown in Table 3.
[0051] Table 2 Reaction conditions and results of Examples 18-20
[0052]
Embodiment 21~41
[0054] In Examples 21 to 41, no catalyst was added, water was the reaction solvent, the reaction temperature was 60° C., and the substrate was changed. The results obtained are shown in Table 3.
[0055] Table 3 Reaction conditions and results of Examples 21 to 41
[0056]
[0057]
[0058] The structural characterization data of some products are as follows:
[0059]
[0060] Compound (I-1): acetophenone benzenesulfonyl hydrazone: White solid, m.p: 84.5℃-85.5℃. 1 H NMR (400MHz, DMSO-d 6 )δ10.55(s,1H),7.94-7.93(m,1H),7.93-7.92(m,1H),7.68-7.63(m,2H),7.62-7.60(m,3H),7.37-7.36( m, 3H), 2.18(s, 3H); 13 C NMR (101MHz, DMSO-d 6 )δ153.93,139.50,137.79,133.45,129.89,129.51,128.83,127.99,126.42,14.79.HRMS(ESI)M / Zcalcd for C 14 H 15 N 2 O 2 S.[M+H] + :275.0849.Found:275.0855.
[0061]
[0062] Compound (I-2): acetophenone p-toluenesulfonyl hydrazone: White solid, m.p: 320.5℃-321.5℃. 1 H NMR (400MHz, DMSO-d 6 )δ10.52(s,1H),7.82(d,J=8.2Hz,2H),7.63-7.61(m,2H),7.41(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com