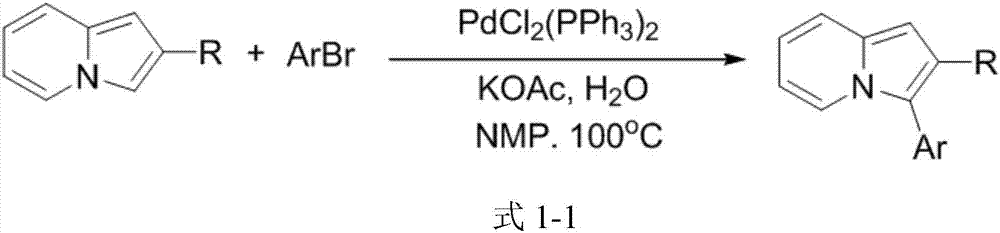
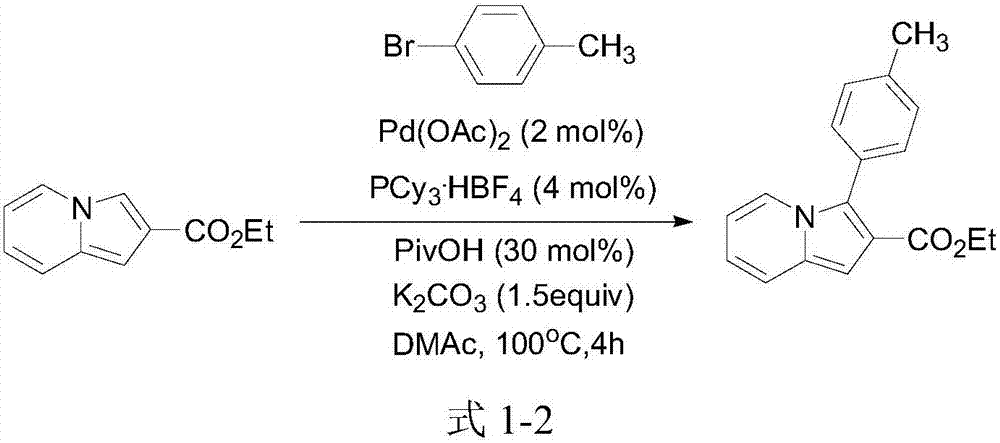
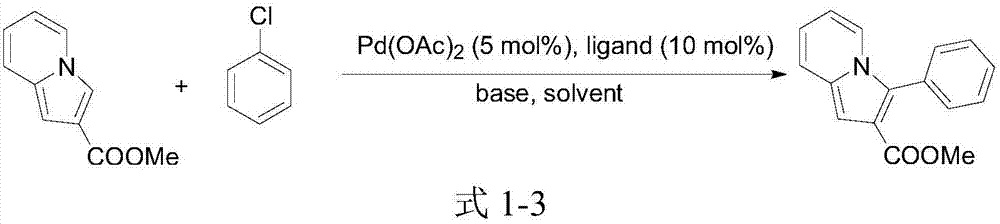A kind of 3-aryl indoxazine acetate derivative and its preparation method and application
A technology of aryl indoleazine acetate and indoleazine acetate, which is applied in the field of 3-aryl indoleazine acetate derivatives and their preparation, and can solve difficult aryl chloride bonds and poor chemical reactivity and other problems, to achieve the effect of mild reaction conditions, good reaction effect, and stable and easy-to-obtain reaction reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] (1) Preparation of indolezine: first slowly add bromoacetic acid (50mmol) to a solution of pyridine (50mmol) in ethyl acetate (30mL), stir the mixture at room temperature for 3 hours, filter and dry to obtain white solid N-(carboxymethyl base) pyridinium bromide. N-(carboxymethyl)bromopyridinium salt (10mmol, 2.04g), vinyl acetate (30mmol), triethylamine (1.5mL), active manganese dioxide (80mmol) were reacted in toluene (80mL) at 90°C for 2 hours , TLC detects the reaction, after the reaction is completed, the solid is filtered off and washed with acetone, the combined organic phase is spin-dried, and the mixture is separated by column to obtain indolezine acetate.
[0040] (2) Indoleazine arylation reaction: Indoleazine acetate (0.3mmol), sodium aryl sulfinate (0.6mmol), catalyst (0.03mmol), ligand (0.05mmol), oxidant (0.3mmol) Mix in acetonitrile (2mL), react at 100°C for 6h under nitrogen protection, after TLC detects that the reaction is complete, cool to room temp...
Embodiment 1~20
[0043] The catalysts of Examples 1-20 were palladium acetate, without adding ligands, and reacted with different oxidants. The reaction results are shown in Table 1.
[0044] The reaction conditions and the result of table 1 embodiment 1~20
[0045] Example
Embodiment 21~37
[0047] The oxidant of Examples 21-37 was tert-butyl peroxyacetate, and the catalyst and ligand were changed. The results obtained are shown in Table 2.
[0048] The reaction conditions and the result of table 2 embodiment 21~37
[0049] Example
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com