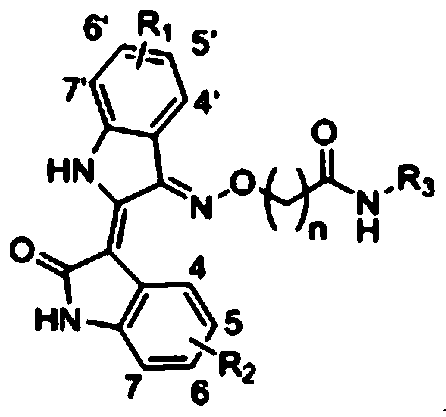
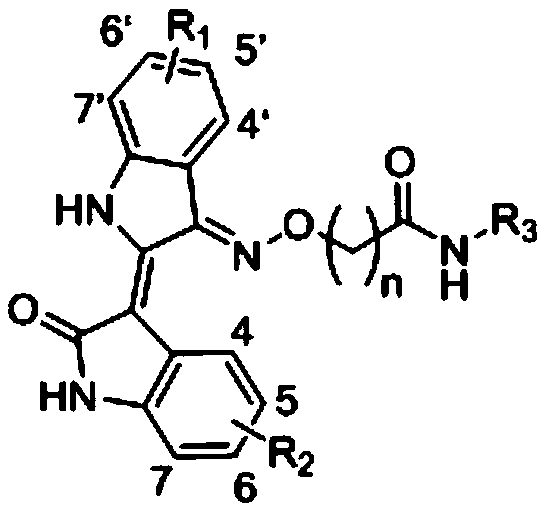
Indirubin derivative and application of indirubin derivative used as dual-target inhibitor of CDK/HDAC
A derivative, indirubin technology, is applied to indirubin derivatives and its application field as a DK/HDAC dual target inhibitor, and achieves the effects of low cost, cheap synthetic raw materials, and obvious anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
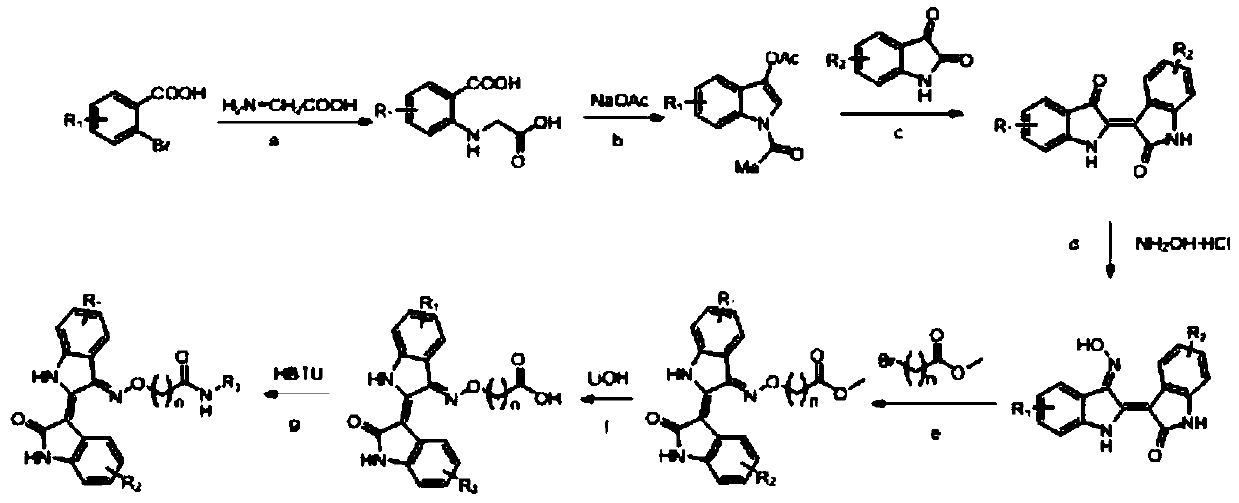
[0027] The following synthetic routes to indirubin derivatives were employed:
[0028]
[0029] Among them, the definitions of n, R1, R2, and R3 are shown in Table 1:
[0030] Table 1
[0031]
[0032] Compounds 1-6 13 C-NMR, 1 H-NMR and HR-MS data
[0033] Compound 1: 1 H NMR (400MHz, DMSO-d 6 ): δ11.70(s,1H),10.80(s,1H),10.40(s,1H),8.71(s,1H),8.61(d,J=7.7Hz,1H),8.11(d,J= 7.1Hz, 1H), 7.42(s, 2H), 7.15(t, J=7.5Hz, 1H), 7.05–6.98(m, 2H), 6.90(d, J=7.4Hz, 1H), 4.58(s, 2H), 2.03-1.96(m, 2H), 1.90(s, 2H), 1.66–1.55(m, 2H), 1.47(d, J=6.2Hz, 2H). 13 C NMR (100MHz, DMSO-d 6 ):171.39,169.51,151.61,145.98,144.51,139.18, 133.31,128.68,126.90,123.74,122.87,122.04,121.12,116.69,112.31,109.51, 100.55,76.86,49.12,32.72,28.85,25.53.HR-MS( ESI) m / z calculated C 22 h 21 N 4 o 4 - [M-H] - for 405.1568, found 405.1582.
[0034] Compound 2: 1 H NMR (400MHz, DMSO-d 6):δ11.71(s,1H),10.83(s,1H),10.40(s,1H),8.61(d,J=7.8Hz,2H),8.10(d,J=7.6Hz,1H),7.41 (s,2H),7.14(t,J=7.5Hz,1H...
Embodiment 2
[0041] CDK Inhibitory Activity and HDAC Inhibitory Activity of Indirubin Derivatives as CDK / HDAC Dual Target Inhibitors in Vitro
[0042] HDAC Inhibitory Activity Screening
[0043] Add the solution required for the reaction system into the EP tube (see Table 2 for details), and finally add the nucleated extract and incubate at 37°C for 1 hour, (add the second sample after adding the first sample at an interval of 30 seconds), after the reaction is complete, Take one on ice in 30s, then add 2×trypsin and continue to incubate for 1h. After the reaction is completed, take one on ice in 30s, then take 120ul and add it to a 96-well plate, Ex: 360nm, Em: 460nm to measure fluorescence (note the whole process dark operation). Finally, S-curve fitting was carried out on the inhibition rate % and concentration of different concentrations of compounds, and the IC50 value was calculated.
[0044] Table 2
[0045]
[0046]*Unit: μL; Substrate: (Boc-Lys(acetyl)-AMC; SAHA final concen...
Embodiment 3
[0059] Antitumor activity of indirubin derivatives as CDK / HDAC dual target inhibitors against different tumor cells.
[0060] The anti-proliferation activity of candidate compounds on five human cancer cell lines was evaluated by the tetramethylazolium blue colorimetric method (MTT), which is recognized for large-scale anti-tumor drug screening and cytotoxicity assay. The test compounds were compounds 2 and 4; the negative control group was the no-drug group; the positive control drug was Vorinostat (SAHA), a clinically used antitumor drug.
[0061] Cell lines: human breast cancer cell MCF-7, human liver cancer cell HepG2, human lung cancer cell A549, human colon cancer cell SW480, and cervical cancer cell Hela.
[0062] Cell proliferation inhibition rate=(OD value of negative control group-OD value of drug group)*100% / OD value of negative control group. The IC was calculated by the inhibition rate of the compound concentration series 50 Value (in μM), the results are shown ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com