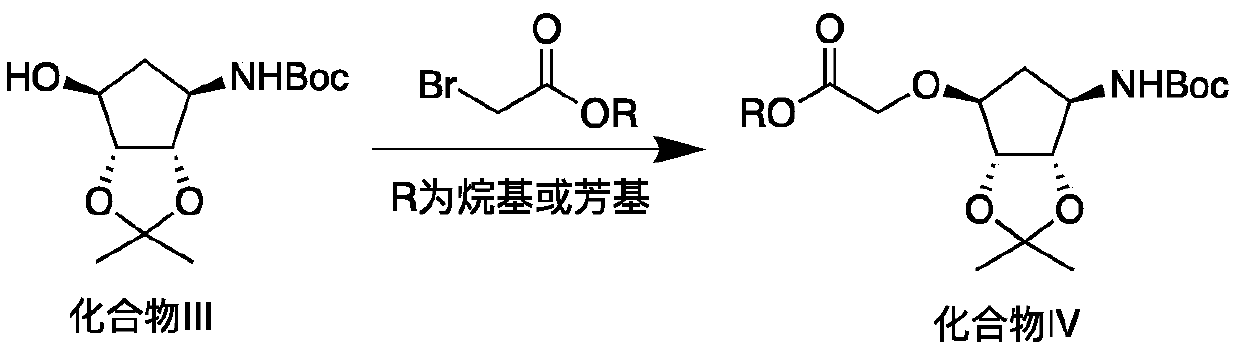
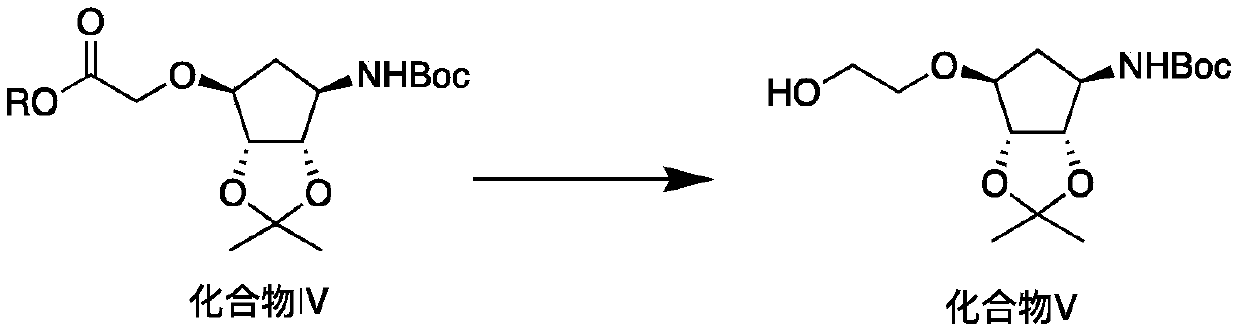
Production method of key intermediate of ticagrelor
A technology of ticagrelor and an intermediate, applied in the field of pharmaceutical synthesis chemistry, can solve the problems of large pollution, high production cost, long synthesis route and the like, and achieve the effects of shortening reaction steps, reducing production cost, and economical and environmental protection in the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
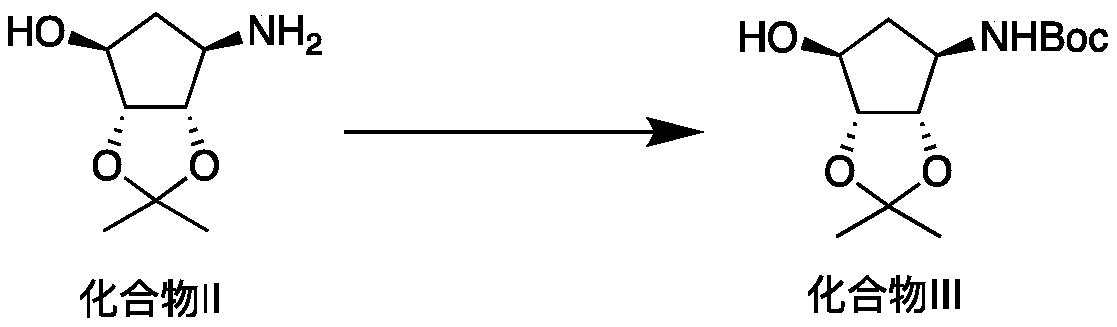
[0042] Preparation of compound III
[0043]865 g of compound II, 690 g of potassium carbonate, 1000 mL of water and 1000 mL of ethyl acetate were added to a 5 L four-neck flask, and 1200 g of di-tert-butyl dicarbonate was added dropwise in an ice bath. After stirring at room temperature for 6 hours, the layers were separated, and the aqueous layer was extracted with 500 mL of ethyl acetate. The combined organic layers were dried and spin-dried, and then recrystallized with n-heptane to obtain 1256 g of compound III with a yield of 92%.
[0044] 1 H-NMR (CDCl 3 ,500MHz)δ5.48(d,J=7.4Hz,1H),4.59-4.48(m,2H),4.25(s,1H),4.07(brs,1H),2.55(brs,1H),2.20(brs ,1H),1.67(d,J=14.4Hz,1H),1.44(s,9H),1.40(s,3H),1.27(s,3H);
[0045] 13 C-NMR (CDCl 3 ,126MHz)δ155.18,110.19,86.23,79.41,56.74,35.49,28.43,26.23,23.85;
[0046] HRMS(ESI):m / zcalcd for C 13 h 23 NO 5 [M+H] + 274.3365,found: 274.3369.
Embodiment 2
[0048] Preparation of compound III
[0049] 865 g of compound II and 2000 mL of water were added to a 5 L four-necked flask, and 1200 g of di-tert-butyl dicarbonate was added dropwise under ice cooling. After stirring at room temperature for 6 hours, the mixture was kept in an ice bath for 2 hours. The resulting solid was filtered with suction, washed with water and dried in vacuo to obtain 1284 g of compound III with a yield of 94%.
Embodiment 3
[0051] Preparation of compound III
[0052] 865 g of compound II and 2000 mL of n-hexane were added to a 5 L four-necked flask, and 1200 g of di-tert-butyl dicarbonate was added dropwise under ice cooling. Stir at 50°C for 5 hours, lower the temperature, filter the resulting solid with suction, wash with n-hexane and dry in vacuum to obtain 1270 g of compound III with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com