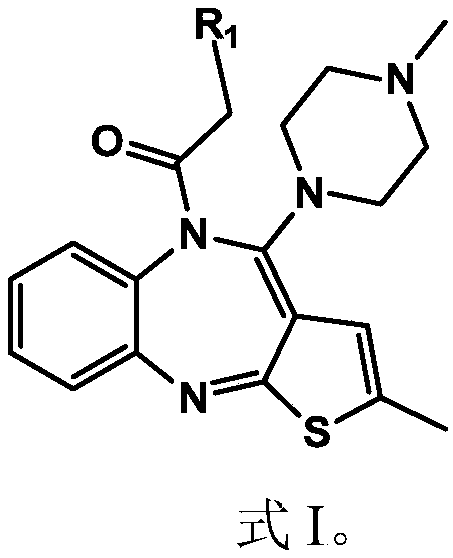
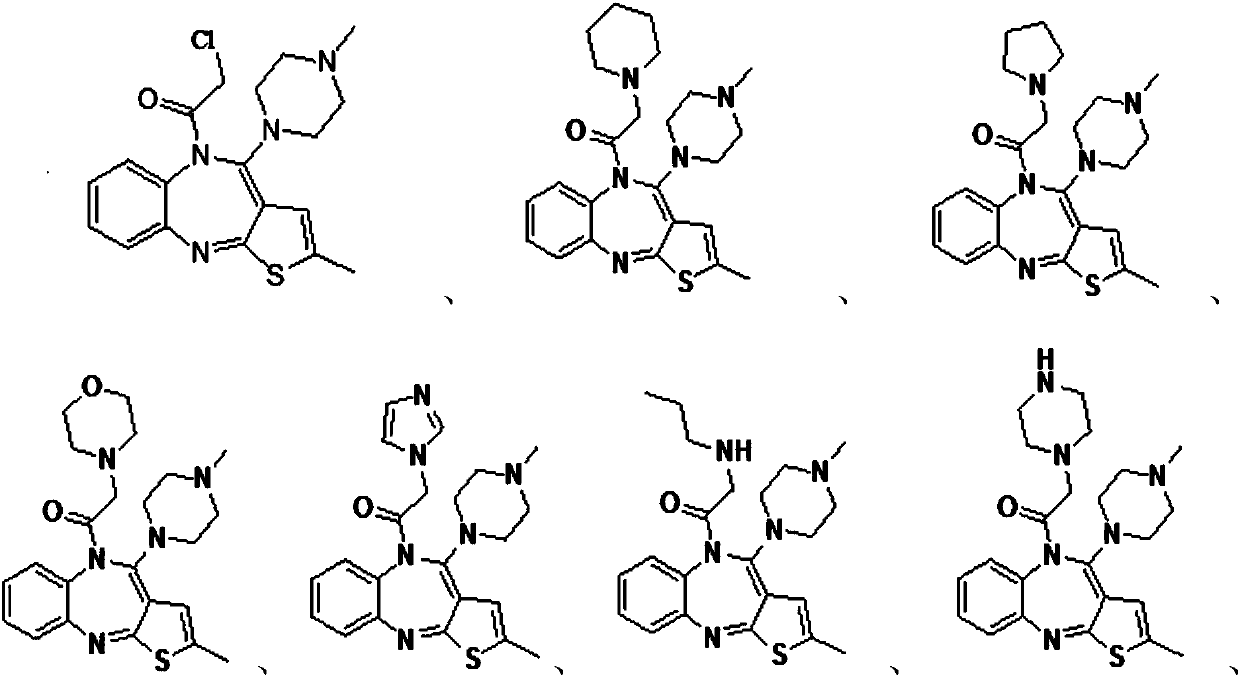
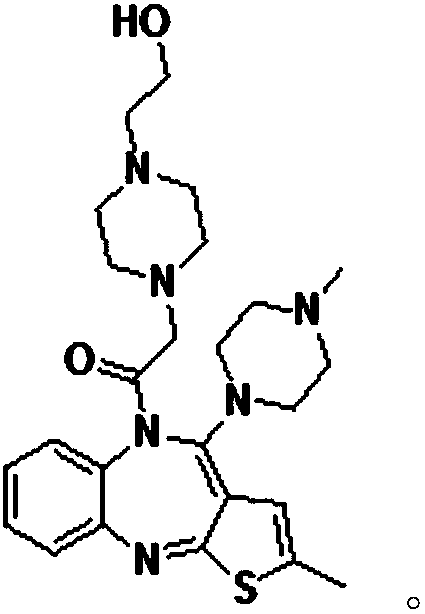
Olanzapine derivative, preparation method and uses thereof
A derivative, olanzapine technology, applied in the field of olanzapine derivatives, can solve the problem of high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthesis of embodiment 1 olanzapine derivative intermediate formula II
[0019] N 2 Under protection, the raw materials olanzapine (1.00g, 3.2mmol, 1eq), triethylamine (4.49mL, 32mmol, 10eq), and tetrahydrofuran (10mL) were sequentially added into a three-necked flask equipped with a thermometer and a stirrer. Cool in an ice bath to 5°C, add dropwise a solution of chloroacetyl chloride (0.510mL, 6.4mmol, 2eq) in tetrahydrofuran (5mL), monitor by TLC until the reaction is complete, stop the reaction, filter the reaction solution with suction, recover the solvent under reduced pressure, and use the residual solution for After washing with saturated sodium bicarbonate solution (30 mL) three times, the organic layer was taken and separated by column chromatography to obtain an intermediate, yellow powder, yield 40%, m.p.245-247°C.
[0020]
[0021] Synthesis of formula 1 intermediate formula II
[0022] Scheme 1Synthesis of intermediates
Embodiment 2
[0023] The preparation of embodiment 2 compound 1
[0024]
[0025] Add intermediate formula II (0.200g, 0.51mmol, 1eq), potassium carbonate (0.352g, 2.55mmol, 5eq), hexahydropyridine (1.01mL, 10.2mmol, 20eq) in a there-necked flask equipped with a thermometer and a stirrer, Tetrahydrofuran (20 mL) was used as a solvent, and the oil bath was heated to reflux. The completion of the reaction was monitored by TLC spotting, and the reaction was stopped. The solvent was recovered by rotary evaporation to obtain a yellow oil. Dichloromethane (10mL) dissolved the oily matter, washed with distilled water (30mL) three times, took the organic layer, recovered the solvent by rotary evaporation, and separated by column chromatography (ethyl acetate:methanol=20:1) to obtain the olanzapine hexahydropyridine derivative , yellow powder, yield 80%, m.p.262-263℃; 1 H NMR (400MHz, CDCl 3 )δ: 7.22(d, J=7.9Hz, 1H), 7.16(d, J=7.0Hz, 1H), 7.11(d, J=7.4Hz, 1H), 7.03(t, J=7.4Hz, 1H) ,6.45(s,1H),...
Embodiment 3
[0026] The preparation of embodiment 3 compound 2
[0027]
[0028] Add olanzapine derivative intermediate formula II (0.200g, 0.51mmol, 1eq), potassium carbonate (0.352mg, 2.55mmol, 5eq), tetrahydropyrrole (0.850mL, 10.2 mmol, 20eq), tetrahydrofuran (20mL) was used as a solvent, heated to reflux, and the reaction was monitored by TLC spotting, the reaction was stopped, cooled and left standing, and the solvent was recovered by rotary evaporation to obtain a yellow oil. Dichloromethane (10mL) dissolved the oil, washed three times with distilled water (30mL), took the organic layer, concentrated, and separated by column chromatography (ethyl acetate: methanol: 20:1) to obtain olanzapine tetrahydropyrrole derivatives, yellow Powder, yield 71%, melting point 235-235°C; 1 H NMR (400MHz, CDCl 3 )δ: 7.25 (d, J = 7.5Hz, 1H), 7.17 (dd, J = 14.3, 7.8Hz, 2H), 7.06 (t, J = 7.5Hz, 1H), 6.48 (s, 1H), 3.54 ( ddd, J=26.1, 22.5, 17.2Hz, 6H), 2.68–2.28(m, 14H), 1.88–1.66(m, 4H); 13 CNMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com