Conjugate for inhibiting melanin synthesis, and application of conjugate in medicines and cosmetics
A technology for inhibiting melanin and conjugates, applied in the field of biomedicine, can solve the problems of low whitening efficiency, the effect needs to be further improved, and the composition is complex.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of polypeptide-compound conjugates
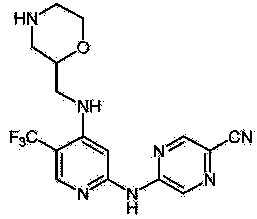
[0030] The compound of formula (I) of the present invention was synthesized according to the synthetic route of the compound in CN104302635A. Add 1mmol of the compound of formula (I), 1mmol of O-benzotriazole-N,N,N',N'-tetramethylpirouentetrafluoroborate (TBTU), 5ml of DMF, nitrogen protection, drop Add 0.5mmol) N,N-diisopropylhexylamine (DIEA), stir at 25°C for 1.5h, add 0.1g targeting membrane-penetrating active peptide TMT, stir at 25°C for 2h, and terminate the reaction. After confirming the correct molecular weight using MS-IT-TOF, the crude product was purified using HPLC. The structural formula of the conjugated product obtained through structural analysis is as follows:
[0031] .
Embodiment 2
[0032] Example 2 MTT method to detect the proliferation of B16 cells
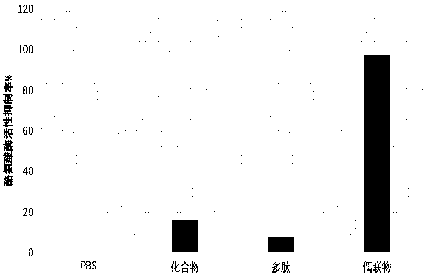
[0033] B16 cells in the logarithmic growth phase were taken, digested with 0.05% trypsin + 0.53 mmol / L EDTA solution, and prepared with fresh culture medium at a density of 6×10 3 cells / mL cell suspension, seeded in 96-well cell culture plate, 180 μL per well, in CO 2Incubate overnight in the incubator. Four sample mass concentration gradients were set for each sample, and each gradient was repeated 3 times. In a 96-well cell culture plate, add the sample diluent and supplement it with PBS to 20 μL, so that the final mass concentration of the samples in the cell culture plate (respectively compound, polypeptide, and conjugate) is 1 ug / L, 1 mg / L, 10 mg / L, 100 mg / L; add 20 μL PBS to the blank control well, and mix well. Place the cell plate in CO 2 After incubation in the incubator for 48 h, the culture solution was sucked off, and 100 μL of PBS and 10 μL of 5 mg / mL MTT solution were added to each well,...
Embodiment 3
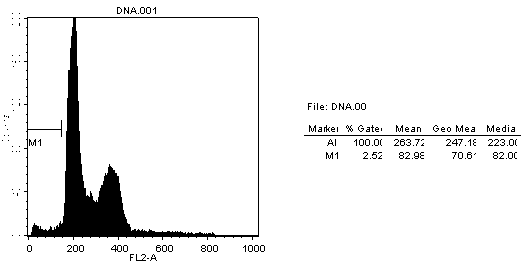
[0037] Example 3 Detection of cell apoptosis by flow cytometry: PI single staining method
[0038] B16 cells in the logarithmic growth phase were taken, digested with 0.05% trypsin + 0.53 mmol / L EDTA solution, and prepared with fresh culture medium at a density of 6×10 3 cells / mL cell suspension, seeded in 96-well cell culture plate, 180 μL per well, in CO 2 Incubate overnight in the incubator. Four sample mass concentration gradients were set for each sample. In a 96-well cell culture plate, add the sample diluent and supplement it with PBS to 20 μL, so that the final mass concentration of the samples in the cell culture plate (respectively compound, polypeptide, and conjugate) is 1 ug / L, 1 mg / L, 10 mg / L, 100 mg / L; add 20 μL PBS to the blank control well, and mix well. Place the cell plate in CO 2 After incubation in the incubator for 48 h, the culture medium was aspirated, centrifuged at 500 r / min for 10 min, and the culture medium was discarded. Wash once with 3ml PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com