Method for preparing mesoporous nano ferroferric oxide particles from titanium dioxide byproduct ferrous sulfate
A technology of ferric oxide and ferrous sulfate, applied in ferrous oxide, iron oxide/iron hydroxide, nanotechnology, etc., can solve the problem of high cost of ferric oxide particles, and achieve battery and capacitor capacity Obvious, the battery and capacitor capacity increase, the effect of the synthesis method is stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Dissolve the refined ferrous sulfate in deionized water according to the molar ratio of 0.2, and dissolve urea in deionized water according to the molar ratio of 0.2, then pour the two solutions into a beaker, stir and mix the reaction evenly, and the volume of the mixed solution 30% of the volume of the beaker;
[0035] (2) Seal the beaker containing the mixed liquid, leaving only one air outlet and one air inlet, and at the air inlet, inject oxygen at a flow rate of 80mL / min for 30 minutes;
[0036] (3) Seal the air inlet and outlet;
[0037] (4) Heat the sealed beaker containing the mixed solution to 85°C for 5 hours. After the reaction, the solid product is filtered out, washed 3 times, and vacuum-dried at 60°C to obtain high-crystallinity mesoporous nano-Fe 3 o 4 particles.
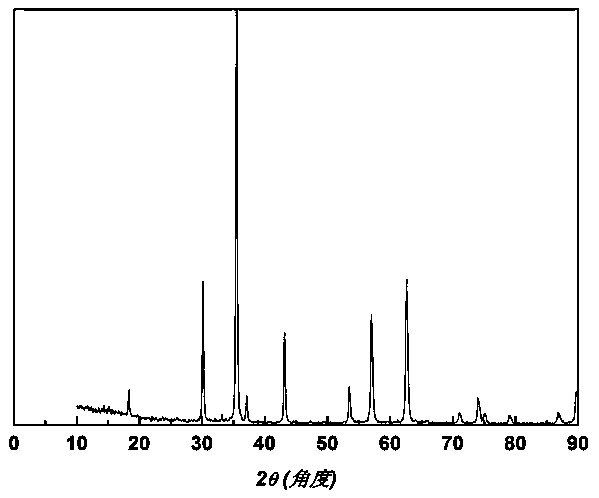
[0038] From figure 1 According to the X-ray diffraction spectrum, we can confirm that the obtained mesoporous nano-Fe3O4 particles belong to spinel (JCPDS: 19-0629), with high crystall...
Embodiment 2
[0045] (1) Dissolve the refined ferrous sulfate in deionized water according to the molar ratio of 0.1, and dissolve the ammonium bicarbonate in deionized water according to the molar ratio of 0.08, then pour the two solutions into a beaker, stir and mix the reaction evenly, and mix The liquid volume accounts for 45% of the volume of the beaker;
[0046] (2) Seal the beaker containing the mixed liquid, leaving only one air outlet and one air inlet, and at the air inlet, flow oxygen at a flow rate of 20mL / min for 30 minutes;
[0047] (3) Seal the air inlet and outlet;
[0048] (4) Heat the sealed beaker containing the mixed solution to 30°C for 5 hours to react. After the reaction, the solid product is filtered out, washed 3 times, and vacuum-dried at 60°C to obtain low-crystallinity mesoporous nano-Fe3O4 particles.
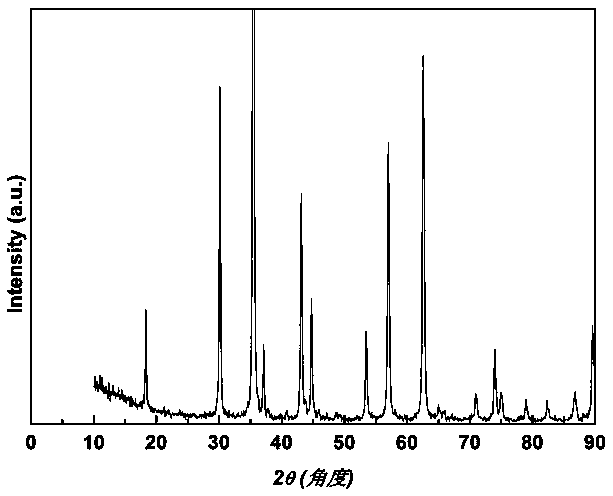
[0049] From figure 2 X-ray diffraction spectrum, we can determine the mesoporous nano-Fe 3 o 4 The particles belong to the spinel type (JCPDS: 19-0629), the ...
Embodiment 3
[0054] (1) Dissolve 0.3 mole of refined ferrous sulfate in deionized water, dissolve sodium bicarbonate in deionized water according to the molar ratio of 0.3, then pour the two solutions into a beaker, stir and mix the reaction evenly, and the volume of the mixed solution accounts for 50% of the volume of the beaker;
[0055] (2) Seal the beaker containing the mixed liquid, leaving only one air outlet and one air inlet, and at the air inlet, inject oxygen at a flow rate of 80mL / min for 30 minutes;
[0056] (3) Seal the air inlet and outlet;
[0057] (4) Heat the sealed beaker containing the mixed solution to 65°C for 15 hours. After the reaction, the solid product is filtered out, washed 3 times, and vacuum-dried at 60°C to obtain high-crystallinity mesoporous nano-Fe 3 o 4 particles.
[0058] From the X-ray diffraction spectrum, we can determine that the obtained mesoporous nano-Fe 3 o 4 The particles belong to the spinel type (JCPDS: 19-0629), with a pure phase and a h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com