Cysteine-containing saccharide derivative and preparation method thereof
A technology of cystine sugars and derivatives, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of difficult application, lack of biocompatibility of synthetic polymers, etc., and achieve stable synthesis methods. , the effect of enriching functionality and expanding the scope of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention provides a preparation method of cysteine-containing carbohydrate derivatives, comprising the following steps:
[0048] (1) 2-amino-2-methyl-1,3-propanediol reacts with dicarbonyl di-tert-butyl and sodium bicarbonate, and after-treatment obtains the first compound;
[0049] (2) the first compound that step (1) obtains generates Williamson ether synthesis reaction with propargyl bromide and potassium hydroxide, and aftertreatment obtains the second compound;
[0050] (3) the second compound obtained in step (2) is esterified with triethylamine and acryloyl chloride, and the aftertreatment obtains the third compound;
[0051] (4) Thiol-ene click chemical reaction occurs between the third compound obtained in step (3) and N-acetyl-L-cysteine methyl ester and dimethylphenylphosphine, and the fourth compound is obtained after post-processing;
[0052] (5) The fourth compound obtained in step (4) undergoes a CuAAC click chemistry reaction with acetyl-...
Embodiment 1
[0071] This embodiment provides a preparation method of cysteine-containing carbohydrate derivatives.
[0072] (1) Preparation of the first compound
[0073] Take 2-amino-2-methyl-1,3-propanediol (10g, 95.11mmol) and add it to a 500mL dry reaction flask, add 100mL methanol and 25mL tetrahydrofuran mixed solution to dissolve, then dropwise add dicarbonyl di-tert-butyl ( 21.87 mL, 95.11 mmol), stirred in an ice bath for 30 min, added sodium bicarbonate (7.99 g, 95.11 mmol), and reacted at 0°C for 24 h. After the reaction, the solvent was removed by rotary evaporation, ethyl acetate was dissolved, added dropwise to petroleum ether for recrystallization, and 19.0 g of white solid was obtained by filtration, and the yield was 90%.
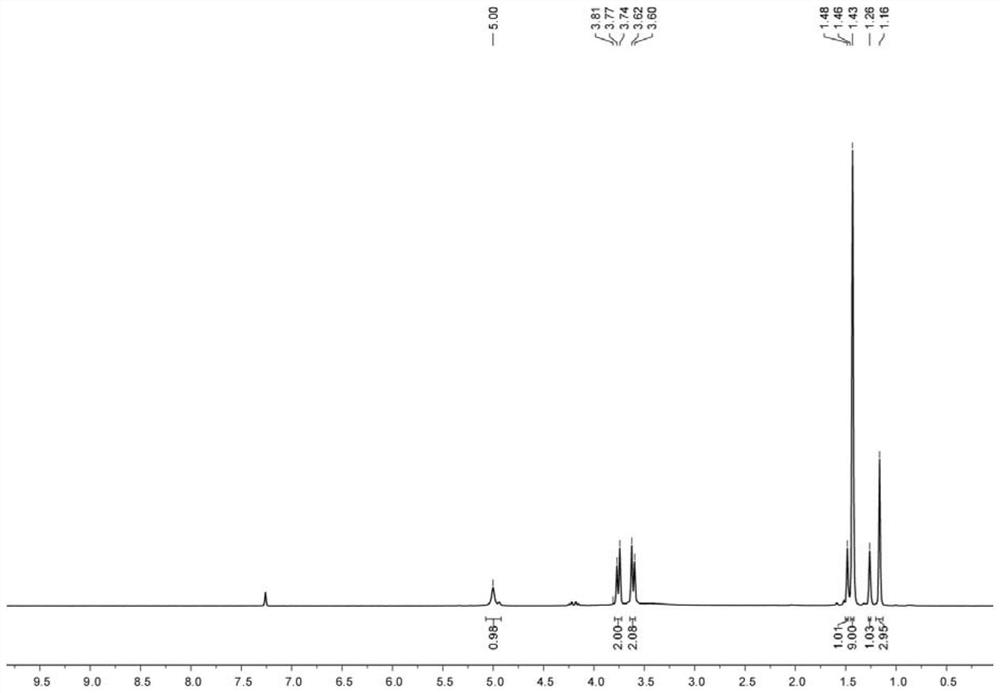
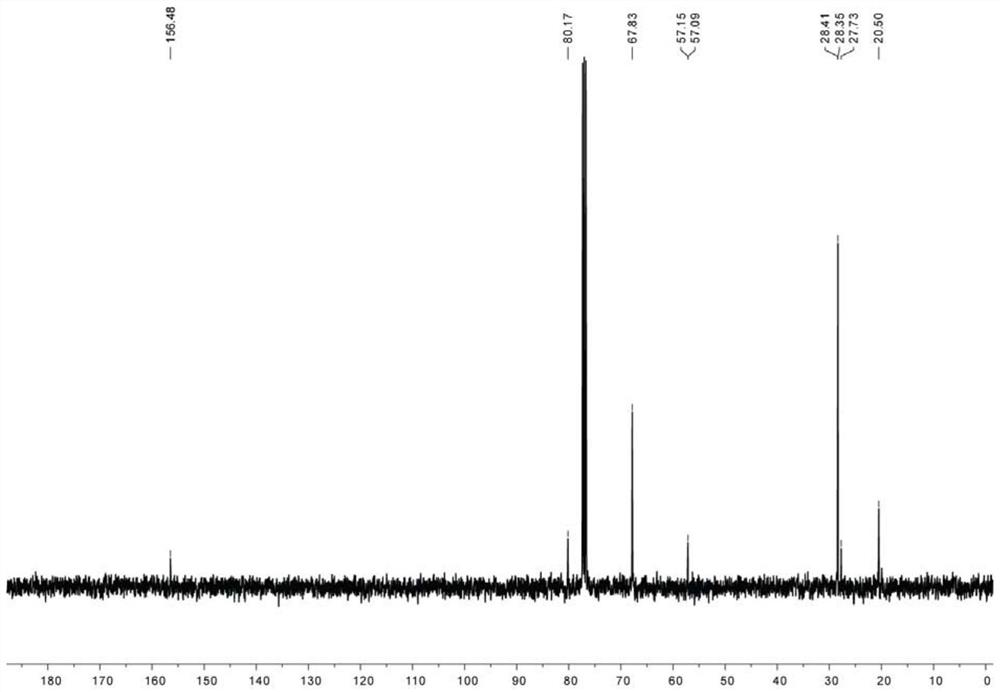
[0074] For the H NMR spectrum and C NMR spectrum of the first compound, please refer to figure 1 and figure 2 shown.
[0075] The chemical structural formula of the first compound is shown below:
[0076]
[0077] The NMR data for the first com...
Embodiment 2
[0108] This embodiment provides a preparation method of cysteine-containing carbohydrate derivatives.
[0109] (1) Preparation of the first compound
[0110] Take 2-amino-2-methyl-1,3-propanediol (10g, 95.11mmol) and add it to a 500mL dry reaction flask, add 100mL methanol and 25mL tetrahydrofuran mixed solution to dissolve, then dropwise add dicarbonyl di-tert-butyl ( 27.26 mL, 118.88 mmol), stirred in an ice bath for 30 min, added sodium bicarbonate 8.79 g, 104.62 mmol), and reacted at 15°C for 16 h. After the reaction, the solvent was removed by rotary evaporation, dissolved in ethyl acetate, added dropwise to petroleum ether for recrystallization, and filtered to obtain 19.5 g of white solid with a yield of 92%.
[0111] (2) Preparation of the second compound
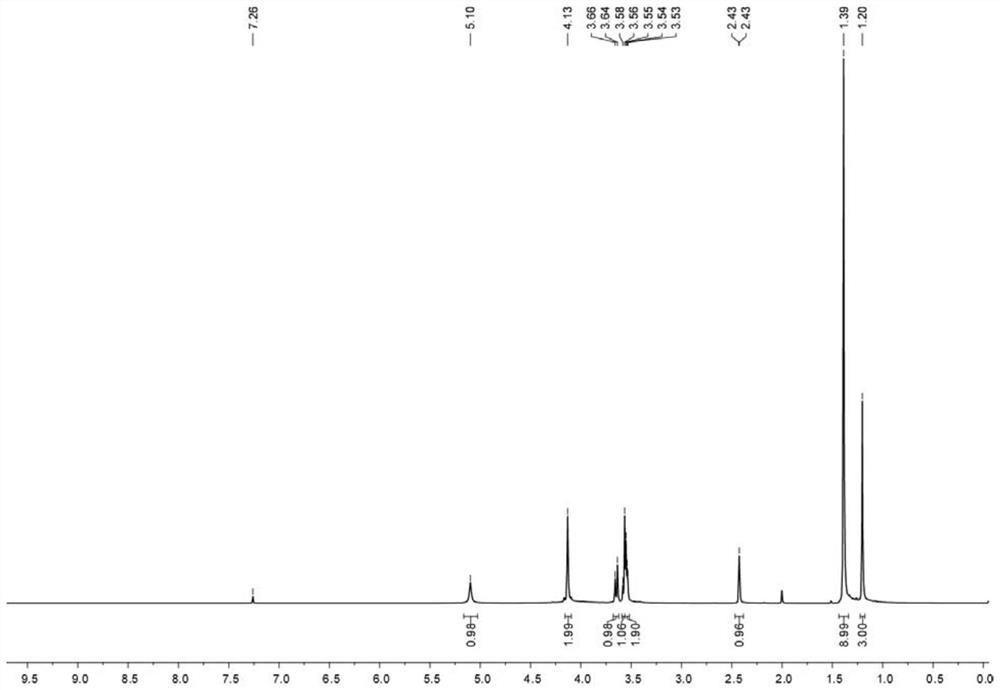
[0112] The first compound (10.00 g, 58.70 mmol) was dissolved in 150 mL of anhydrous DMF, and placed in an ice-water bath to stir and dissolve, and propargyl bromide (6.89 mL, 88.05 mmol) was added slowly dropwise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com