Double-arm heterogeneous sugar-containing compound and preparation method thereof
A compound and double-arm technology, applied in the field of double-arm sugar-containing compound synthesis, can solve the problems of reducing product yield and increasing reaction steps, and achieve the effect of stable synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] The present invention provides a method for preparing a double-armed heterogeneous sugar-containing compound, comprising the following steps:
[0074] (1) aniline substitution reaction occurs with external norbornene dianhydride and p-bromoaniline, and aftertreatment obtains the first compound;
[0075] (2) The first compound obtained in step (1), together with Pd(PPh 3 ) 4 , anhydrous sodium carbonate and 4-hydroxyphenylboronic acid take place Suzuki coupling reaction, and after-treatment obtains the second compound;
[0076] (3) the second compound that step (2) obtains and triethylamine, isopropylidene-2,2-bis (oxymethyl) propionic anhydride and 4-dimethylaminopyridine generate the esterification reaction of hydroxyl and acid anhydride, After treatment, the third compound is obtained;
[0077] (4) The third compound obtained in step (3) and Dowex-H + The hydrolysis reaction of the ketal occurs, and the aftertreatment obtains the fourth compound;
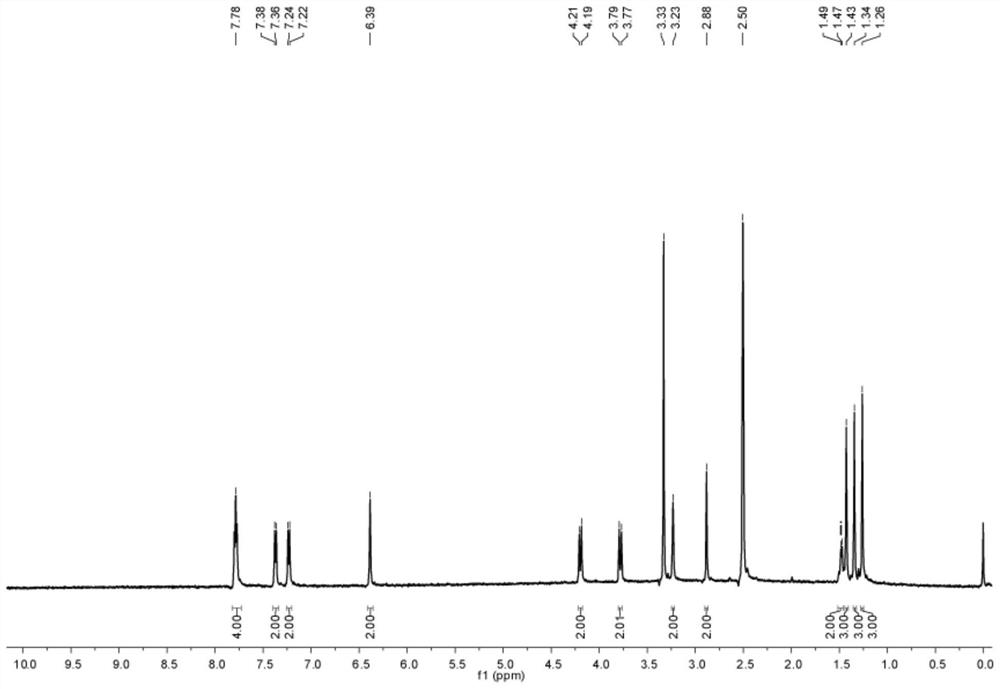
[0078] (5) the...
Embodiment 1
[0114] This embodiment provides a double-armed heterogeneous sugar-containing compound and a preparation method thereof.
[0115] (1) Synthesis of the first compound
[0116] cis-5-norbornene-exo-2,3-dicarboxylic acid anhydride (20 g, 0.122 mol) was placed in a dry round bottom flask, and acetic acid 140 mL was added. The mixture was heated to 120°C until cis-5-norbornene-exo-2,3-dicarboxylic anhydride was dissolved and 4-bromoaniline (25.2 g, 0.146 mol) was added. The reaction was carried out at 120°C for 0.5h. After the reaction was completed, it was poured into ice water to form a white precipitate. The monomer was extracted with dichloromethane, washed with distilled water and dried. 29 g of a white solid (ie, the first compound) was obtained in a yield of 75%.
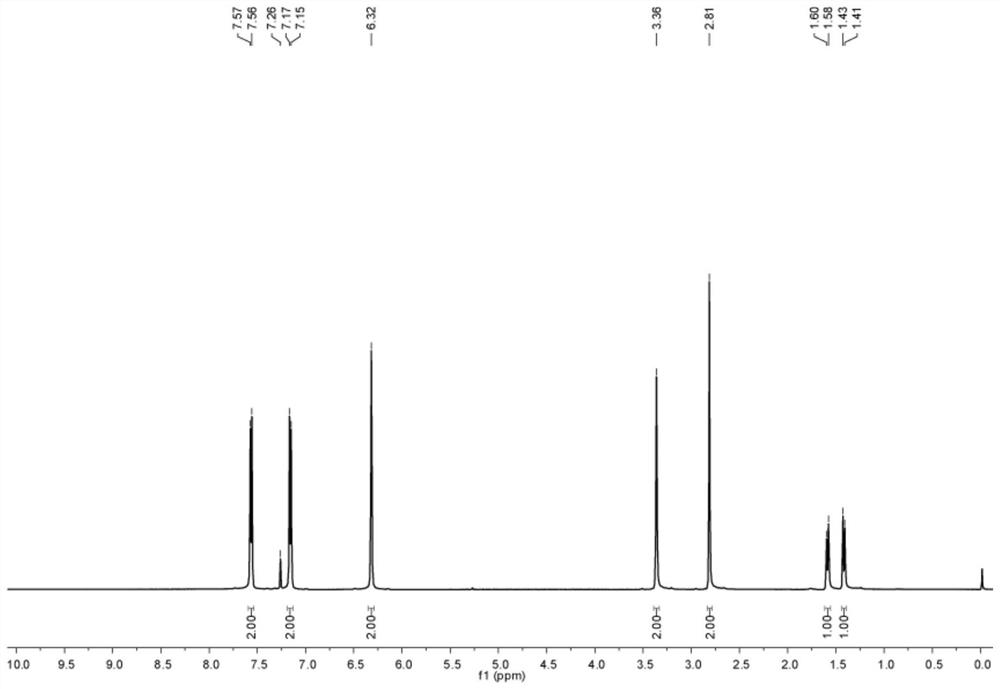
[0117] The H NMR spectrum of the first compound is as follows figure 1 shown;
[0118] The chemical structural formula of the first compound is shown below:
[0119]
[0120] The NMR data for the first c...
Embodiment 2
[0165] This embodiment provides a double-armed heterogeneous sugar-containing compound and a preparation method thereof.
[0166] (1) Synthesis of the first compound
[0167] cis-5-norbornene-exo-2,3-dicarboxylic acid anhydride (20 g, 0.122 mol) was placed in a dry round bottom flask, and acetic acid 140 mL was added. The mixture was heated to 120°C after cis-5-norbornene-exo-2,3-dicarboxylic anhydride was dissolved and 4-bromoaniline (20.74 g, 0.122 mol) was added. The reaction was carried out at 130 °C for 1 h. After the reaction was completed, it was poured into ice water to form a white precipitate. The monomer was extracted with dichloromethane, washed with distilled water and dried. 30 g of a white solid (ie, the first compound) was obtained in a yield of 79%.
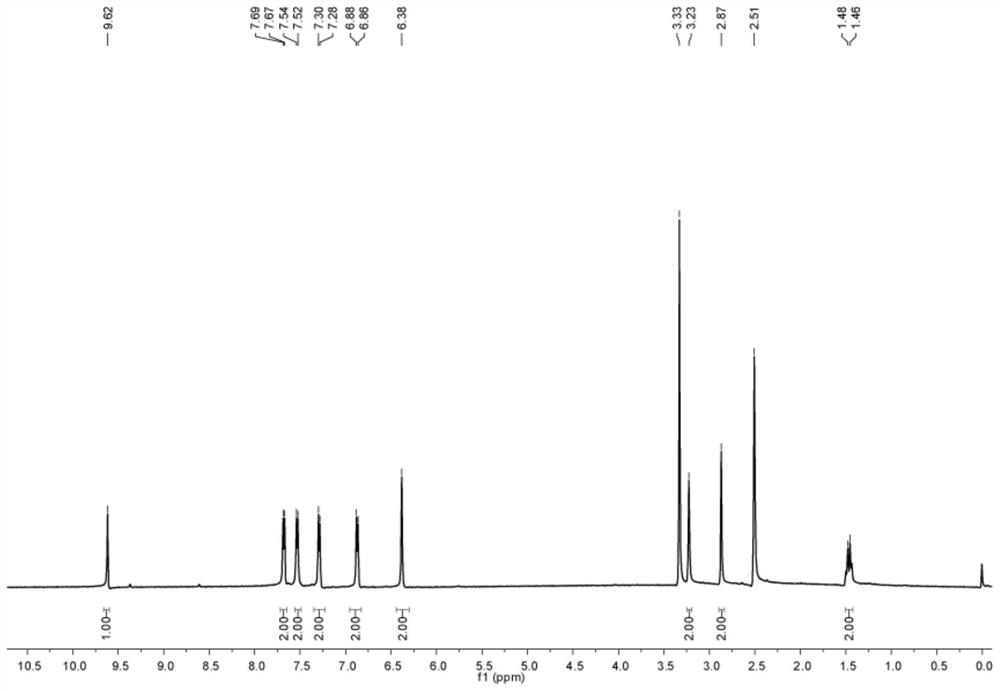
[0168] The chemical structural formula of the first compound is shown below:
[0169]
[0170] (2) Synthesis of the second compound
[0171] A nitrogen purge with the first compound (10 g, 31.4 mmol) and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com