Heterogeneous rearrangement method for preparing caprolactam from cyclohexanone oxime
A technology of cyclohexanone oxime and caprolactam, which is applied to the preparation of lactam, chemical instruments and methods, organic chemistry and other directions, can solve the problems of unsatisfactory solution, unstable process, low heat transfer efficiency, etc., and achieves enhanced reaction process control, The effect of improving the selectivity of the reaction and reducing the temperature rise of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
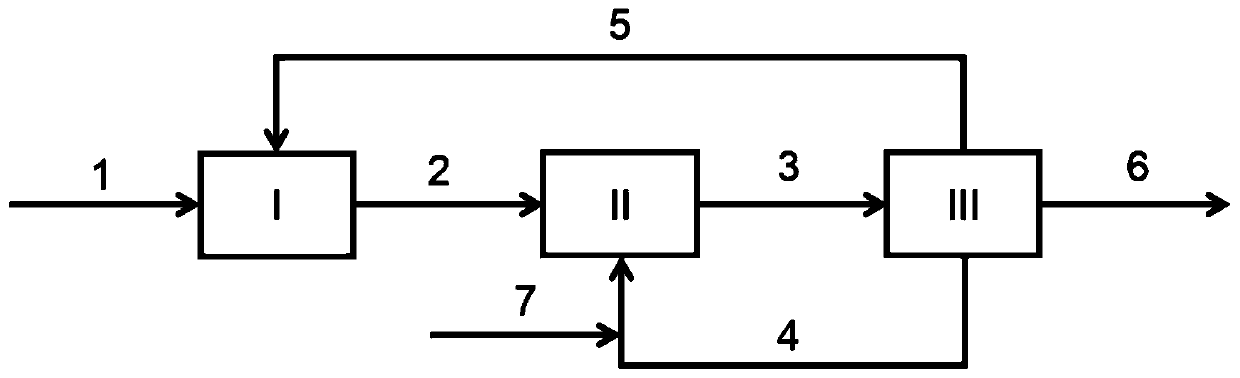
[0040] Experiment according to the method of the present invention, the n-decane solution (2) of the cyclohexanone oxime of 12.5kg / h, wherein cyclohexanone oxime massfraction 8%, with the oleum (7) of the 10% concentration of 1kg / h ) and 2kg / h of the mixed solution of the circulating acid phase (4) are mixed in the microreactor (II), and the inlet temperature is 80°C, and enters the phase separator for phase separation, and the phase separator temperature is 100°C. The reaction acid oxime ratio (that is, the molar ratio of oleum added to cyclohexanone oxime) is 1.2, the circulation ratio is 1, and the total residence time of the reaction is 30 min. Reaction conversion >99.9%, selectivity >99.5%.
Embodiment 2
[0042] Experiment according to the method of the present invention, the D80 solution (2) of the cyclohexanone oxime of 20kg / h, wherein cyclohexanone oxime mass fraction 5%, with the oleum (7) of the 20% concentration of 1kg / h and 1kg The mixed solution of circulating acid phase (4) per hour is mixed in the stirred reactor (II), the inlet temperature is 60°C, and enters the phase separator for phase separation, and the temperature of the phase separator is 100°C. The reaction acid oxime ratio (that is, the molar ratio of adding fuming sulfuric acid to cyclohexanone oxime) is 1.2, the circulation ratio is 0.5, and the total residence time of the reaction is 20 minutes. Reaction conversion >99.9%, selectivity >99.6%.
Embodiment 3
[0044]Experiment according to the method of the present invention, the n-octane solution (2) of the cyclohexanone oxime of 12.5kg / h, wherein cyclohexanone oxime massfraction 8%, with the oleum of 20% concentration of 1.2kg / h ( 7) mixed with 10kg / h of the circulating acid phase (4) in the microreactor (II), the inlet temperature is 80°C, and enters the phase separator for phase separation, the temperature of the phase separator is 100°C. The reaction acid oxime ratio (that is, the molar ratio of oleum added to cyclohexanone oxime) is 1.4, the circulation ratio is 5, and the total residence time of the reaction is 30 minutes. Reaction conversion >99.9%, selectivity >99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com