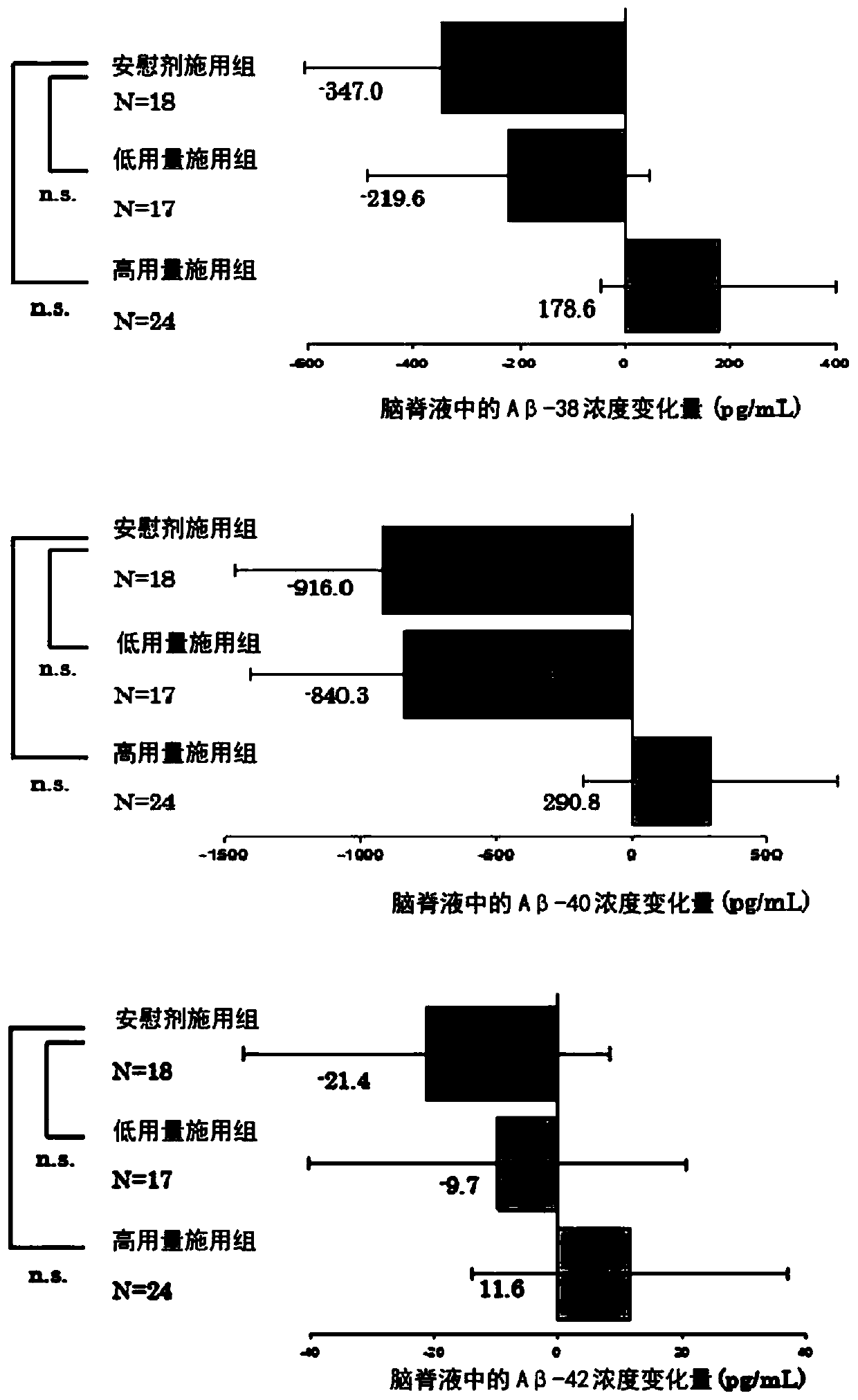
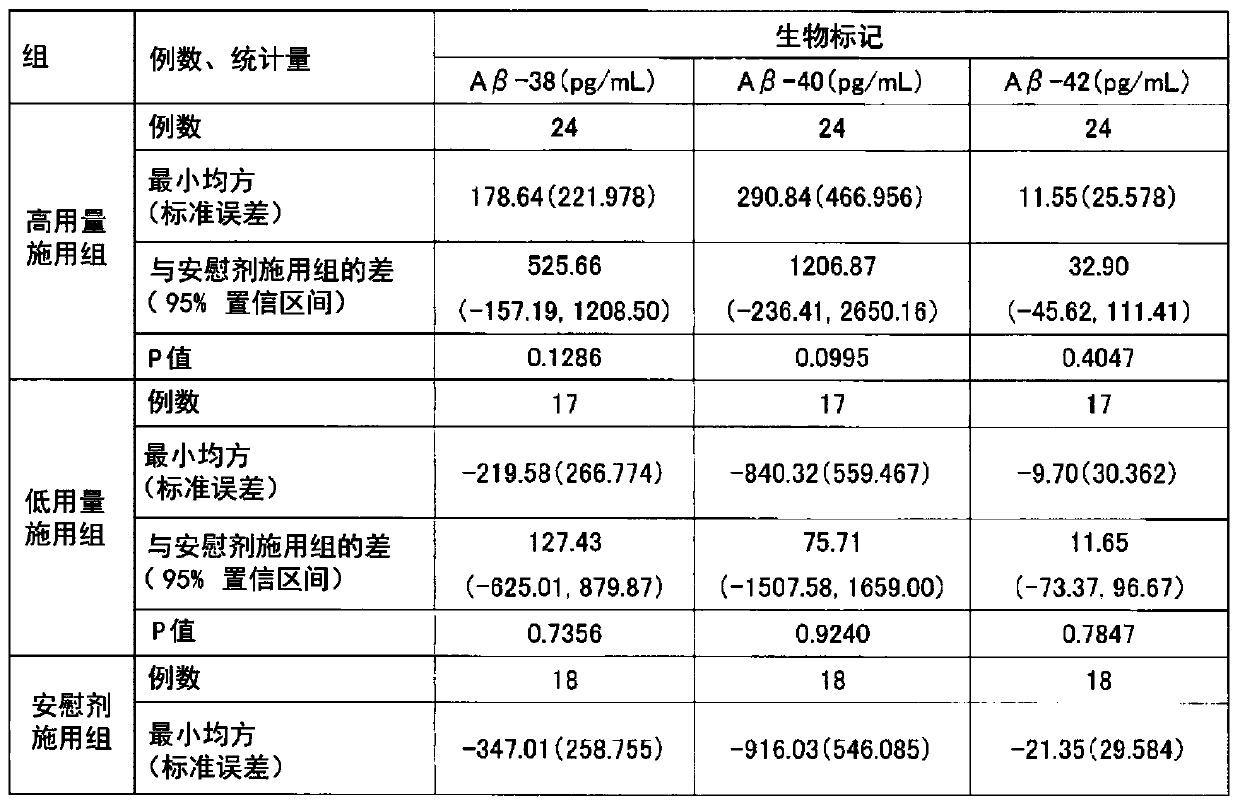
Amyloid-beta protein level decreasing agent
A technology of reducing amyloid protein and agents, which can be used in drug delivery, organic active ingredients, nervous system diseases, etc., and can solve problems such as deterioration of cognitive function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0095]0.9726 g of magnesium stearate (magnesium stearate, Merck) was added to 174.03 g of the maleate salt of compound A, and mixed for 30 minutes. This mixed powder was compressed and molded with a dry granulator (TF-LABO (roller pressure 3 MPa), Freund Industries), and the molded solid was sized. To 60.0 g of the obtained sized powder, 49.51 g of lactose (FlowLac 90, Meggle Japan), 16.50 g of crystalline cellulose (CEOLUS PH302, Asahi Kasei Chemicals) and cross-linked carboxymethyl fiber were added after sieving through a sieve with an aperture of 850 μm. Sodium Primellose (Primellose, DMV Japan) 6.67 g, mixed for 10 minutes. 0.6667 g of magnesium stearate was added to this mixed powder, and it mixed for 30 minutes. For this mixed powder, a tablet press machine (HT-P18A, Hata Tekkosho (Japanese original: バブルアール面) pestle with a tablet diameter of 8.5 mm and a compression pressure of about 12 kN was used. Iron Works)) to perform tablet compression to obtain a 250 mg circular...
preparation example 2
[0097] 60.90 g of mannitol (Parteck M200, Merck) and 3.60 g of croscarmellose sodium were added to 53.70 g of the maleate salt of compound A, and mixed for 10 minutes. 1.80 g of magnesium stearate was added to the mixed powder, and mixed for 30 minutes. This mixed powder was tableted using a pestle with double R sides with a tablet diameter of 8.5 mm at a tableting pressure of about 10 kN to obtain one 250 mg round plain tablet. The plain tablet is coated with a coating agent (Opadry (オパドライ) 03F44057, 00F440000 (hypromellose 2910: 71.5%, Macrogol 6000: 14.166%, talc: 7.167%, titanium oxide: 7.067%) at a rate of 8 mg per tablet. , ferric oxide: 0.1%), Japan Colorcon), add a trace amount of carnauba wax to obtain film-coated tablets.
preparation example 3
[0099] 11.11 g of magnesium stearate was added to 1988.89 g of the maleate salt of compound A, and mixed for 30 minutes. The mixed powder is compressed into a dry granulator, and the formed solid is sized. Add mannitol 26.21g, ethyl cellulose (Ethocel 100FP premium, Dow Chemicals) 7.50g, crystalline cellulose (CEOLUS KG-1000, Asahi Kasei Chemicals) 3.75g, cross-linked polydimensional fiber in 107.13g of the obtained sized powder. Ketone (Kollidon CL-SF, BASF) 3.75g and croscarmellose sodium 0.75g were mixed for 30 minutes. 0.90 g of magnesium stearate was added to the mixed powder, and mixed for 5 minutes. This mixed powder was tableted using a pestle with double R sides with a tablet diameter of 8.5 mm at a tableting pressure of about 7 kN to obtain one 315 mg round plain tablet. The plain tablet was coated with a coating agent at a ratio of 9 mg per tablet, and a small amount of carnauba wax was added to obtain a film-coated tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com