A kind of in vitro expansion and culture method of peripheral blood T cells
A culture method and peripheral blood technology, applied in the field of biomedicine, can solve problems such as disadvantage, increase the burden on patients and CAR-T, and achieve the effect of reducing difficulty, saving initial blood supply, and alleviating serious shortages.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Pre-prepared peripheral blood T cell in vitro expansion medium: use serum-free immune cell culture medium X-VIVO15 (1L / bottle, Lonza, product number: 04-418Q), add 10% FBS (Gibco, product number: 10091- 148), and sequentially added interleukin-2 (IL-2, final concentration 75 μg / L, Sino, product number: 11848-HNAY1) with a concentration of 250 μg / mL, interleukin-15 (IL-15, final concentration 40 μg / L, Sino, Cat. No.: 10360-H07E), 1 mg / mL human CD3 monoclonal antibody (Biolegend, Cat. / L), mixed well as the T cell in vitro expansion medium.
[0041] (2) Collect blood (sodium heparin anticoagulant) using traditional methods and routine blood collection methods, and collect 20 mL of whole blood;
[0042] (3) Ficoll density gradient centrifugation was used to separate the peripheral blood lymphocytes in the above-mentioned whole blood, the method is as follows:
[0043] i) The 1640 culture is based on a 37°C water bath for warming;
[0044] ii) Take out the Ficoll se...
Embodiment 2
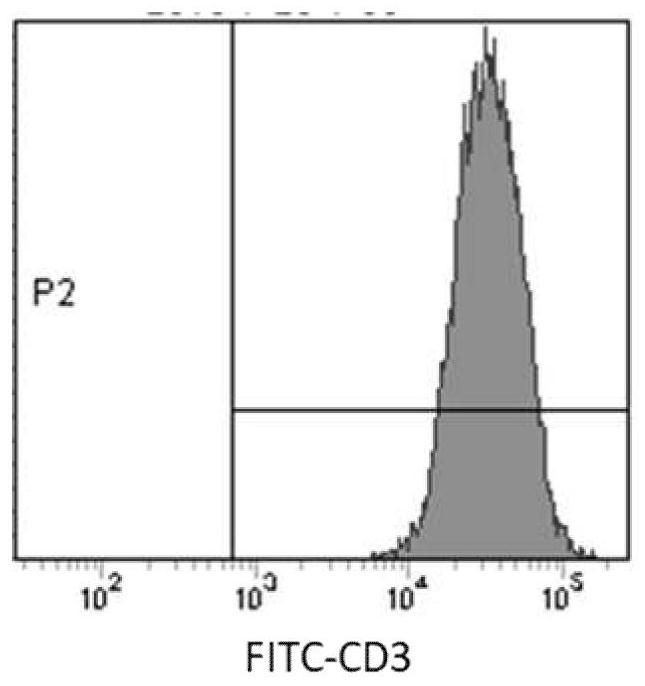
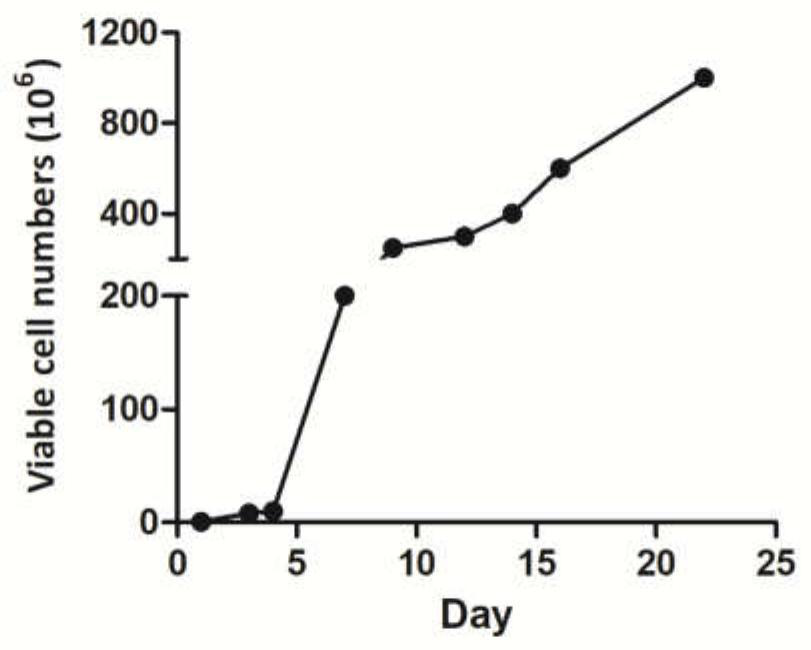
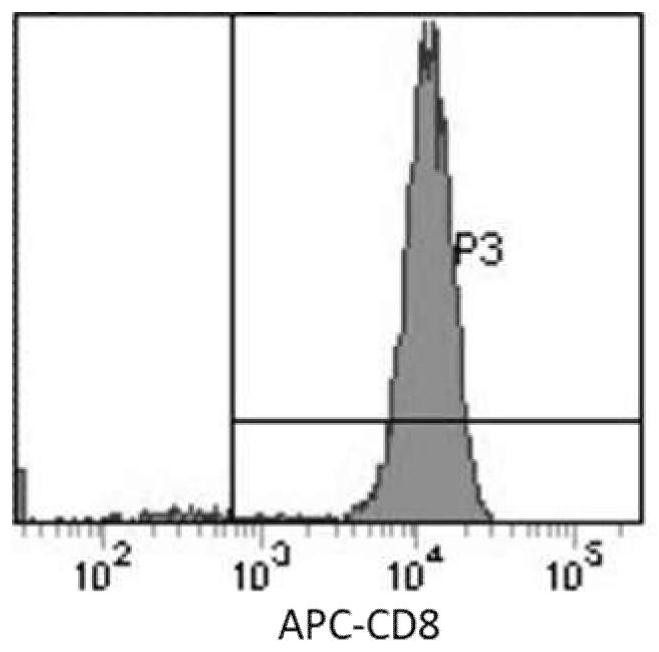
[0056] T cells that had been cryopreserved for 7 days were taken out from liquid nitrogen (5×10 6 ), using the peripheral blood T cell in vitro expansion medium in Example 1 to quickly revive T cells, and to culture at a density of 1 × 10 6 cells / mL to re-stimulate T cells (culture conditions 37°C, 5% CO 2 ), which promotes the reactivation and expansion of T cells. 5×10 after 2-3d 5 -5×10 6 The medium was inoculated at a culture density of 1 cell / mL, and the resuscitated T cells were infected with the lentiviral vector PCDH lentiviral vector to obtain chimeric antigen receptor T cells targeting EGFR (EGFR CAR- T), using the above-mentioned T cell in vitro expansion medium to carry out routine culture of the prepared CAR-T, every 2-3d with 5 × 10 5 -5×10 6 The culture density of cells / mL was inoculated and changed, and it was obtained by cell counting ( Figure 5 ) CAR-T cells can be expanded to 60-80 times the initial amount on the 22nd day in vitro, that is, from the i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com