A class of anti-human EGFR antibody drug conjugates and its preparation method and application
A technology of antibody drugs and antibody-drug conjugates, applied in the direction of antibodies, drug combinations, anti-tumor drugs, etc., to achieve enhanced anti-tumor effect, considerable yield, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthesis of embodiment 1 Linker-MMAE
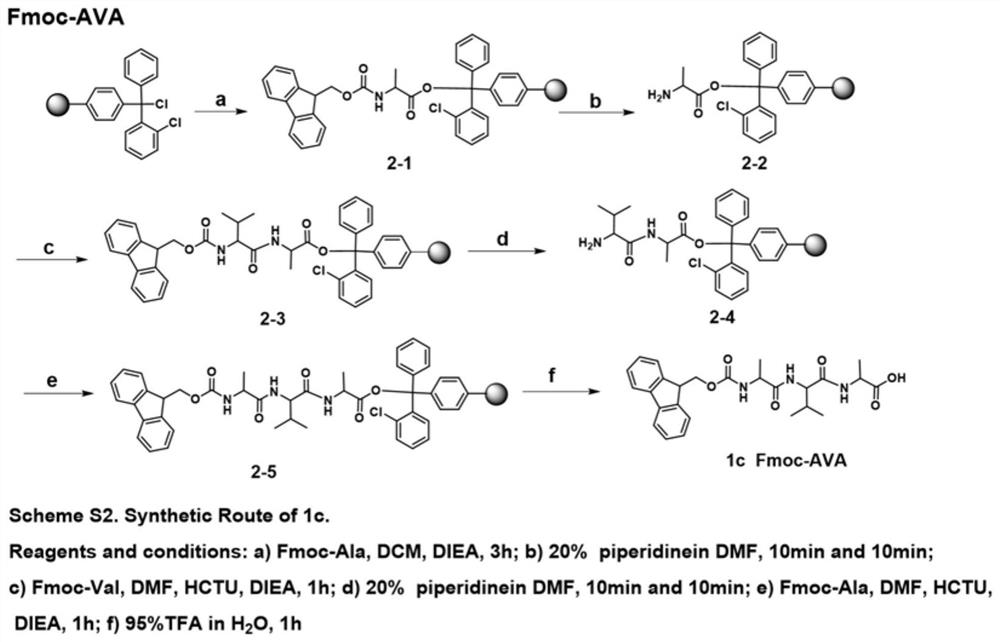
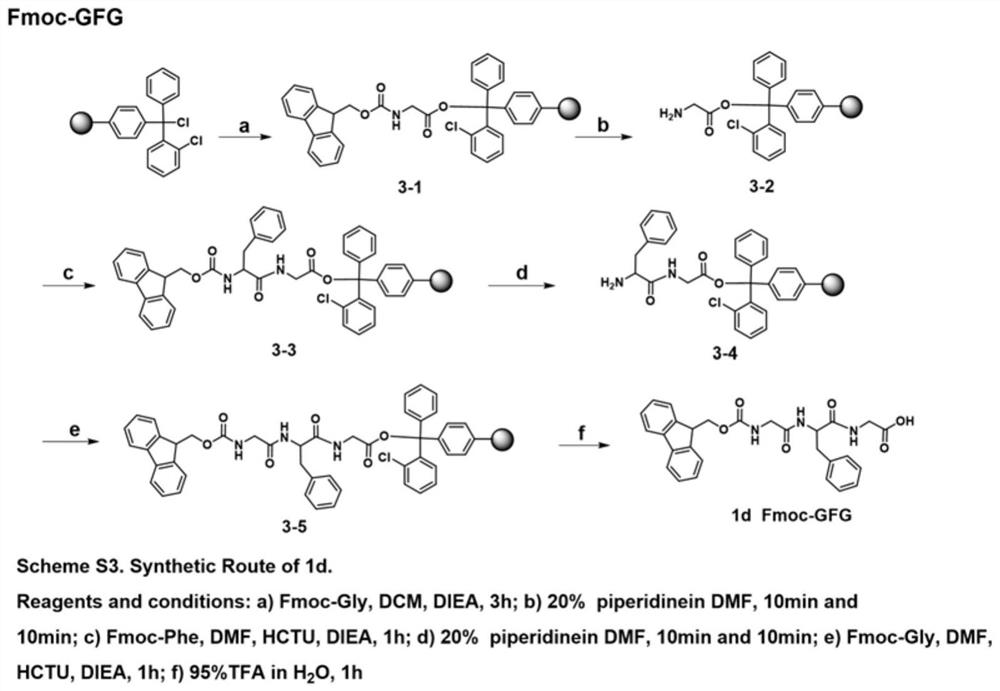
[0059] 1.1 Synthesis of Fmoc-AA
[0060] 1.1.1 Synthesis of Fmoc-dipeptide
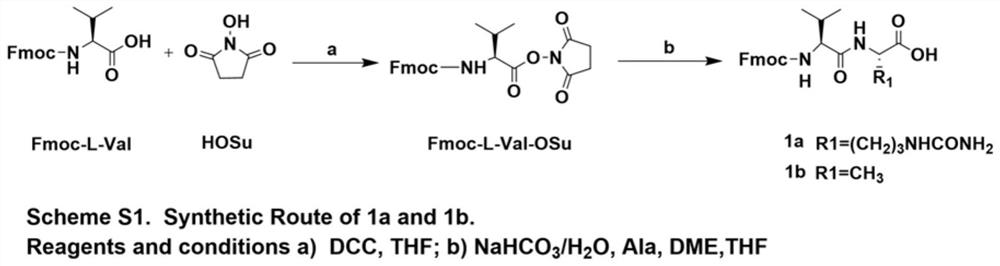
[0061] Synthesis of Fmoc-L-Val-OSu
[0062] according to figure 1 As shown in the Scheme S1 step, the reaction products Fmoc-L-Val (10g, 29.3mmol) and HoSu (3.7g, 32.3mmol) were dissolved in 100mL THF, and DCC (6.6g, 32.3mmol) was added under ice-bath conditions, After the addition was complete, the ice bath was removed, and the reaction was stirred overnight at room temperature. After the reaction was completed (petroleum ether: ethyl acetate = 1:1 detection), the reaction solution was moved to an ice bath, and after the solid was completely precipitated, it was filtered with suction, and the filtrate was rotary evaporated until it became foamy, and the residue was the product. The reaction product was directly used in the next reaction without further purification.
[0063] Synthesis of 1a
[0064] according to figure 1 As shown in Scheme S1,...
Embodiment 2
[0176] Example 2 In vitro plasma stability detection of Linker-MMAE
[0177] Linker-MMAE to be tested was prepared into a 10mM DMSO stock solution for use. 4 μL DMSO stock solution was added to 996 μL blank rat plasma, mixed evenly and placed in a 37°C water bath for incubation. 50 μL samples were taken at 0, 30, 60, 180, and 360 minutes respectively, and the reaction was terminated. Two parallel samples at each reaction time point were stored at -20°C and processed uniformly after the sampling was completed. The sample was centrifuged to settle protein, and the supernatant was taken, and the concentration of MMAE was detected by LC-MS / MS, and the concentration unit (nM) was detected. Draw the curve of the release amount of MMAE as a function of time, and calculate the release rate of MMAE in plasma. The release rate is shown in Table 1.
[0178] The results showed that spacer fraction M-2 was the best (K=4.0 nM / hr), followed by M-3 (K=10.9 nM / hr) and M-1 (K=22.6 nM / hr). Co...
Embodiment 3
[0181] Embodiment 3 Linker-MMAE digestion release rate experiment
[0182] Linker-MMAE to be tested was prepared into a 10mM DMSO stock solution for use. 10 μL of the stock solution was added to 2490 μL of 100 mM L-Cys PBS buffer (pH=6) to prepare a working buffer solution with a concentration of 0.04 mM. Cathepsin B, purchased at a concentration of 1.52 mM, was diluted with PBS buffer at pH=6 to form a 0.4 mM protease solution. 1000 μL of 0.04 mM compound buffer working solution was mixed with 10 μL of 0.4 mM protease solution (the molar ratio of substrate to protease was 1:10), and incubated in a 37°C water bath. The reaction was terminated at 0, 10, 60, 120, 240, and 360 minutes, respectively, and two parallel samples were taken at each reaction time point. The concentration of MMAE was detected by LC-MS / MS, and the detection concentration unit (nM).
[0183] The results are shown in Table 2. M-2 has the fastest hydrolysis release (K=32.3nM / hr), followed by C-4 (K=32.1nM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com