Preparation and application of a class of antineoplastic drugs based on matrix metalloproteinase tissue inhibitor-2
A technology of anti-tumor drugs and matrix metals, applied in the field of biomedicine, to achieve good innovation, good application prospects, and guaranteed biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] "Example 1" construction of recombinant expression vector pHBM-TIBP
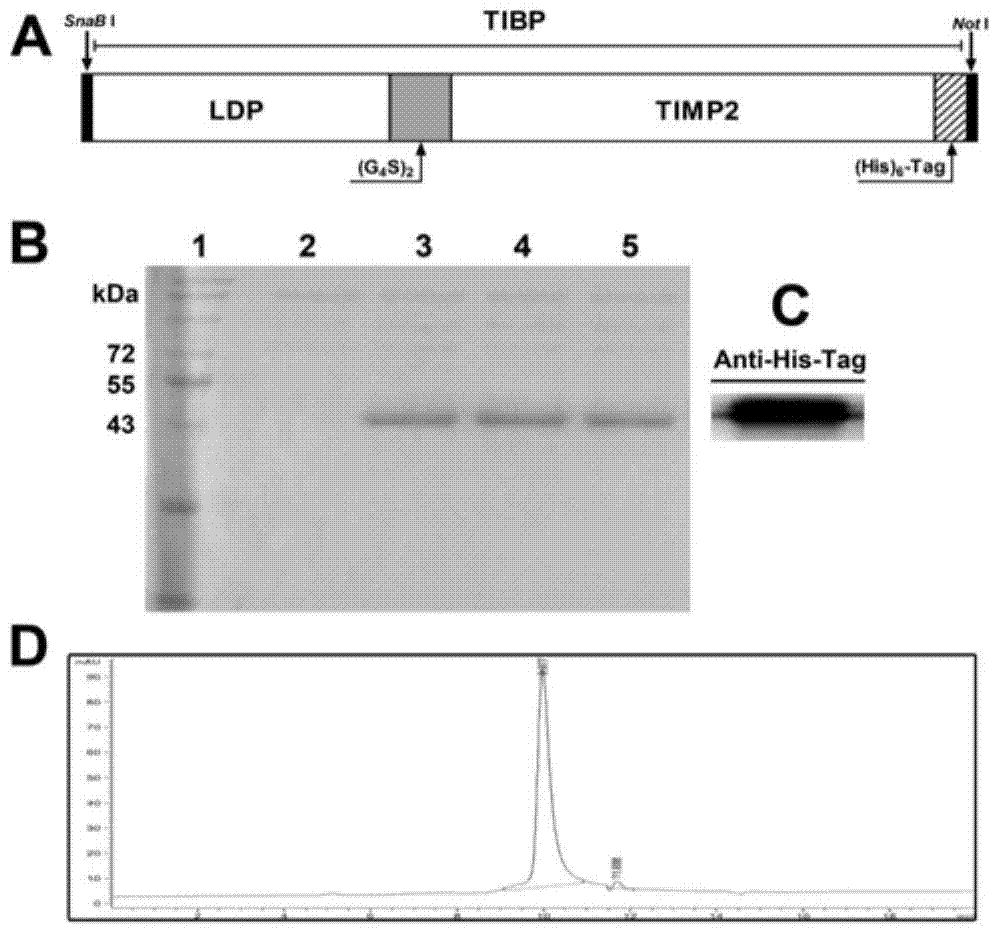
[0041]The TIBP gene sequence composed of lidamycin prosthetic protein LDP and TIMP2 was synthesized by Nanjing KingScript Biotechnology Co., Ltd., and passed OptimumGene TM Codon optimization technology processing. PCR primers used in molecular biology experiments were provided by Invitrogen TM Company Synthesis. The full-length sequence of TIBP mainly includes the nucleotide sequence encoding LDP and the nucleotide sequence encoding TIMP2, which are connected by a flexible linker peptide (G 4 S) 2 Connection, full-length gene sequence ( figure 1 A) Insert into the pHBM-9005 plasmid to obtain the expression vector. After linearization with SalI endonuclease, the target band was recovered, and electrotransformed into Pichia pastoris GS115 competent cells to obtain the expression strain containing the target gene sequence. The strain is named Pichia pastoris GS115-TIBP, and was sent to the General ...
Embodiment 2
[0042] 《Example 2》Induced expression, purification and identification of TIBP
[0043] The strains containing the recombinant expression vectors obtained in Example 1 were used for small-scale induced expression, and high-efficiency expression strains were screened out. The main steps are: pick the single colonies grown on the MD plate and inoculate them into BMGY medium (1% yeast extract, 2% peptone, 1.34% YNB, 4×10 -5 %biotin, 100mM pH 6.0 potassium phosphate buffer, 1% glycerol), 28-30°C, 280-300rpm, cultivated to logarithmic phase (OD 600 =2-6). Transfer 0.5mL of culture solution to 50mL of BMGY medium (500mL shake flask, bottled at ≤10%), culture at 28-30°C, 280-300rpm, for 24-36h. Centrifuge at room temperature (RT) at 3,000×g for 5 minutes, discard the supernatant, collect the cells, and transfer the cell pellet to 50 mL of BMMY medium (1% yeast extract, 2% peptone, 1.34% YNB, 4×10 -5 % biotin, 100mM pH 6.0 potassium phosphate buffer, 1% methanol), 28°C, 280-300rpm, ...
Embodiment 3
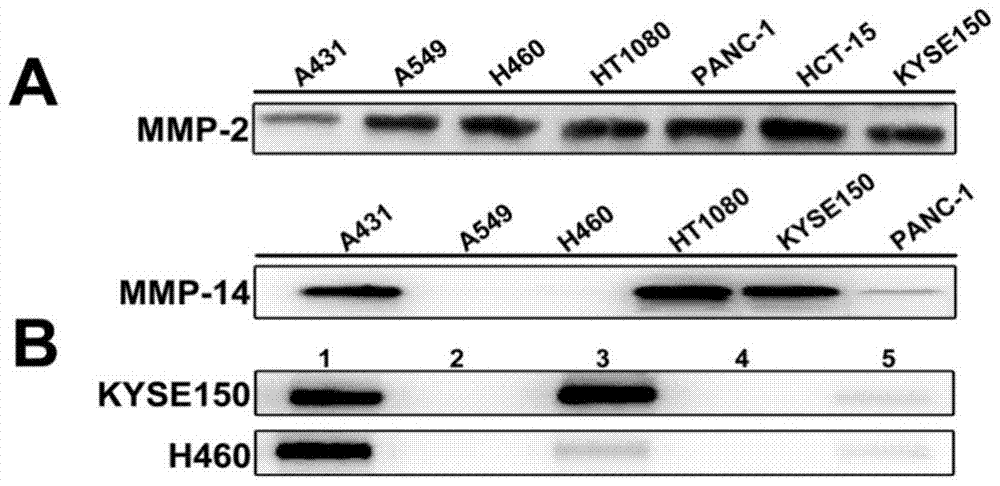
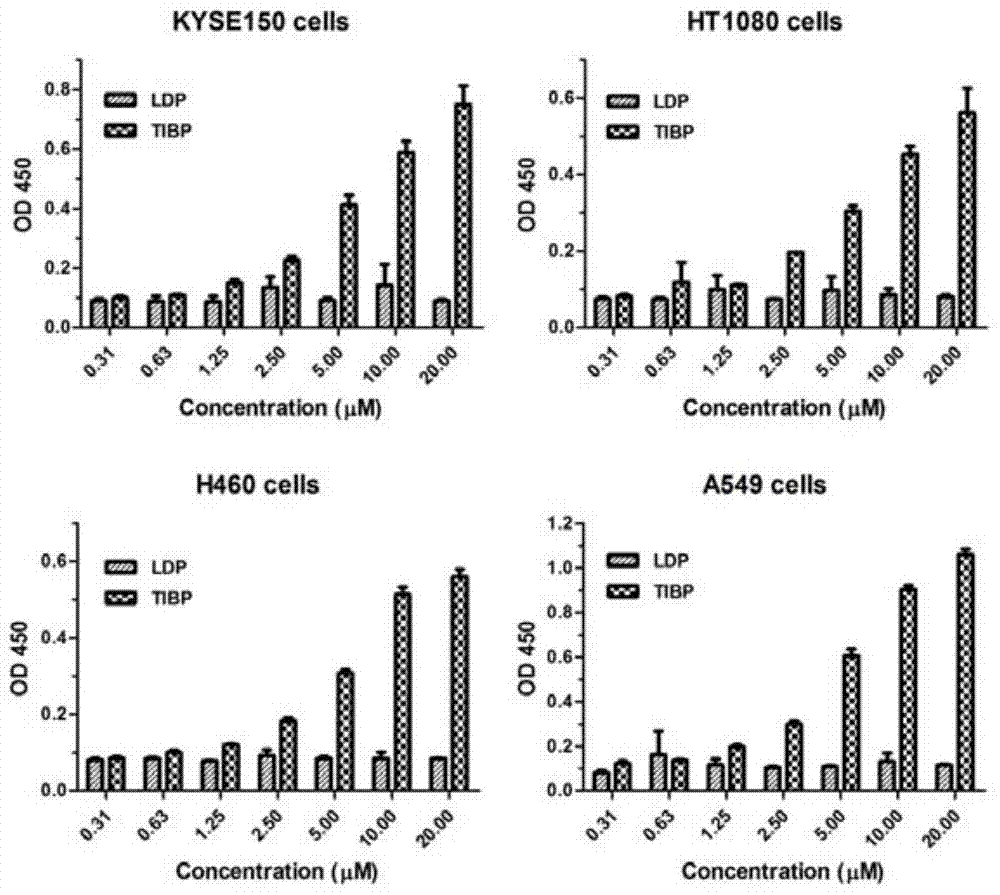
[0044] 《Example 3》Analysis of TIBP and tumor cell affinity
[0045] (1): Western blot detection of MMP-2, -14 expression levels in different tumor cells
[0046] Human squamous cell line A431 in logarithmic growth phase, human non-small cell lung cancer cell lines H460 and A549, human fibrosarcoma cell line HT1080, human pancreatic cancer cell line PANC-1, human colon cancer cell line HCT-15 and human Seven kinds of cells of esophageal squamous cell carcinoma cell line KYSE150, remove the culture medium, rinse twice with PBS, add 150-250 μL of lysate according to the amount of cells in each well of a 6-well plate (high-efficiency RIPA tissue / cell lysate, add 10 μL of PMSF per 1 mL of RIPA (phenylmethylsulfonyl fluoride), to a final concentration of 1 mM), gently pipet several times to make the lysate fully contact with the cells, and lyse on ice for 20 min. The lysed cells were centrifuged at 10,000-14,000×g for 10 min at 4°C, the supernatant was collected, and the protein wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com