Application of combination of natural immune agonist and brain homeostasis regulatory protein in treating Alzheimer's disease
A technology of natural immunity and protein regulation, applied in the field of biomedicine, can solve the problem that the pathological process of TREM2AD is not very clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of STING agonist
[0025] Preparation of cGAMP: cGAMP (cyclized-GMP-AMP) was catalyzed and synthesized by cyclized cGMP-AMP dinucleotide synthetase (cGAS) according to the literature method under activation conditions after binding to DNA. The purity is above 98%. (Pingwei Li, et al., Immunity, 2013, 39(6), 1019-1031.). Thio-cGAMP, c-di-AMP, thio-c-di-AMP, c-di-GMP were purchased from sigma company.
Embodiment 2
[0026] Example 2: Brain Homeostasis Regulation Protein Zn 7 Preparation of MT3gH625
[0027] All biochemical reagents and kits were purchased from Sigama or Invitrogen unless otherwise specified. Zn 7 The preparation of MT3gH625 was carried out according to the literature method. (Wei Xu, Qiming Xu, Hao Cheng, Xiangshi Tan, Scientific reports, 2017, 7(1), 13763.)
[0028] Zn 7 Determination of metal content in MT3gH625
[0029] Take a certain amount of recombined fusion protein Zn 7 MT3gH625 was nitrified overnight at 65°C with a small amount of concentrated nitric acid, diluted 10 times, and the content of metal Zn was measured on an inductively coupled plasma optical emission spectrometer (ICP-OES). The results show that each mole of Zn after recombination 7 The MT3gH625 fusion protein contained 7±0.2 moles of Zn.
[0030] Zn 7 Removal of endotoxin in MT3gH625
[0031] Zn 7 MT3gH625 first uses ultrafiltration membrane to intercept aggregated endotoxin (LPS), and t...
Embodiment 3
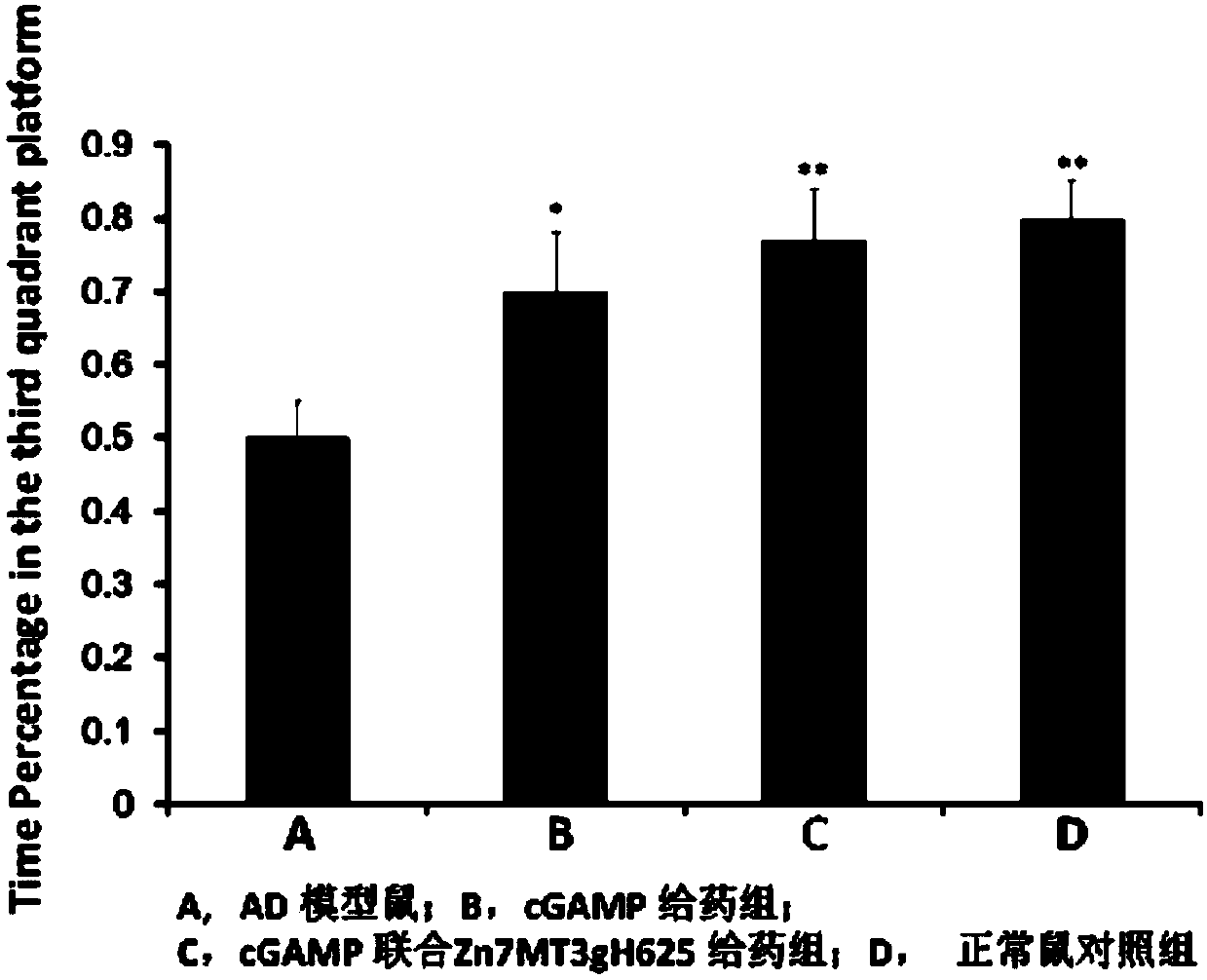
[0032] Example 3: Alzheimer's disease mouse model and STING agonist combined with Zn 7 MT3gH625 drug treatment APP / PS1 transgenic mice were purchased from Beijing Zhongke Zesheng Biotechnology Co., Ltd., 4 months old, weighing 24-26g. Test drug name: STING agonist, STING agonist combined with Zn 7 MT3gH625. Properties: white powder. Vehicle: normal saline. Preparation method: Prepare the solution with the required concentration with physiological saline solution before use. The dosage is: 10mg / kg. Administration method: intraperitoneal injection; administration frequency: once a day for 60 consecutive days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com