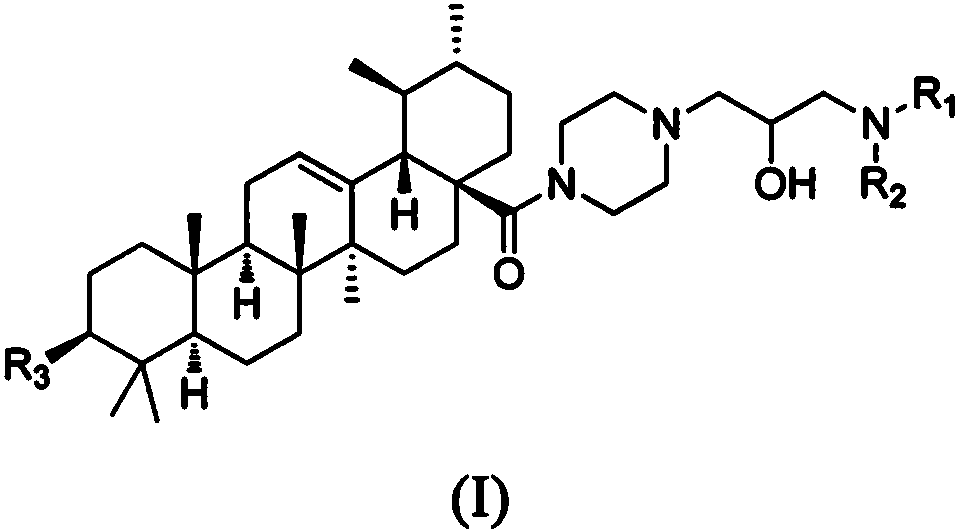
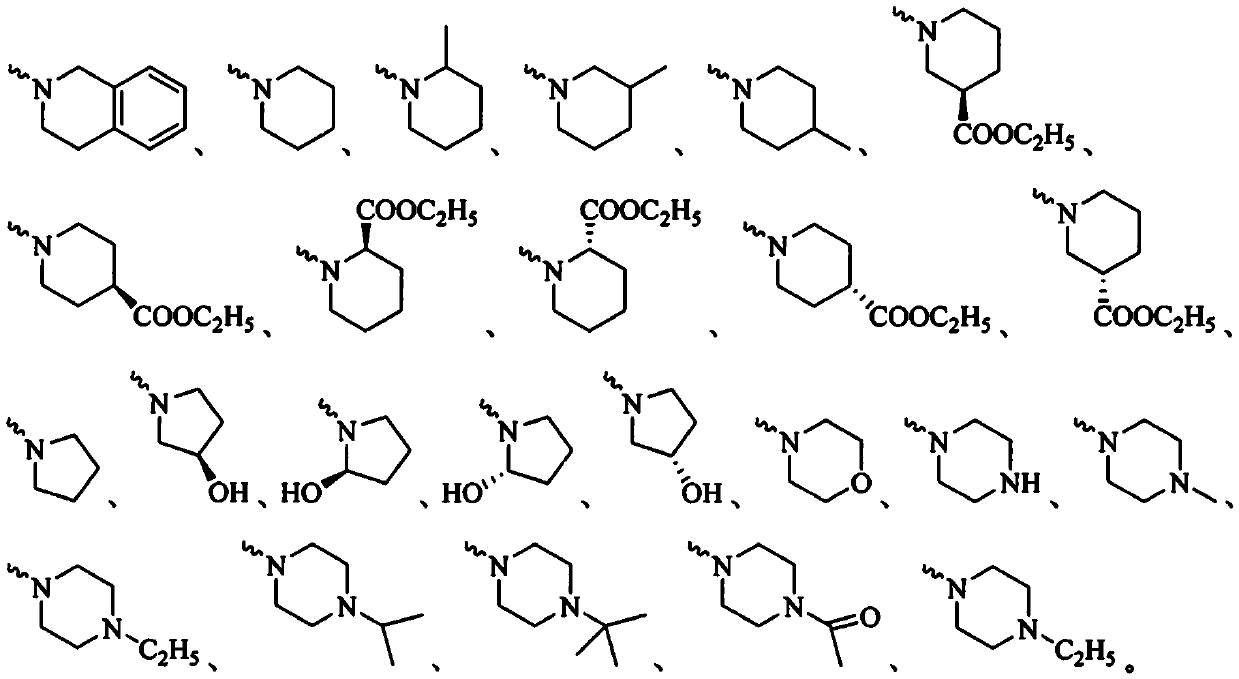
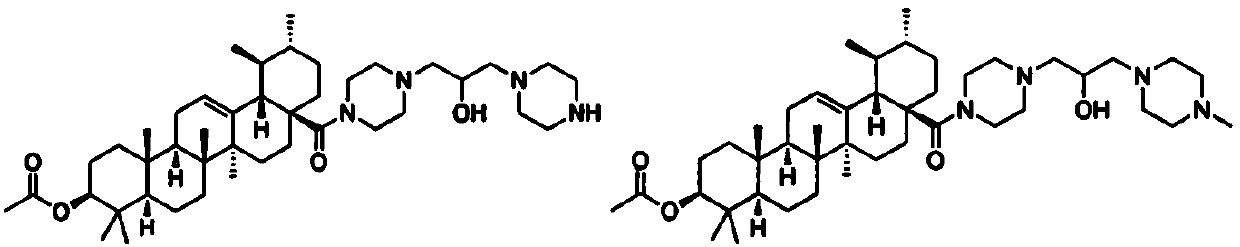
Acetoxyl ursolic acid piperazine compound containing isopropanolamine substructure, and preparation method and application of acetoxyl ursolic acid piperazine compound
A technology of acetoxyursolic acid and propanolamine, which is applied in the field of 3β-acetoxyursolic acid-28-piperazine compounds and its preparation, can solve the problems of low inhibitory concentration and reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the preparation of intermediate 3β-acetoxy ursolic acid
[0056] Add ursolic acid (21.9mmol) to 20mL of anhydrous pyridine dissolved with DMAP (2.6mmol) and acetic anhydride (87.6mmol), stop the reaction after 12 hours at room temperature, remove the solvent, add 50mL of water, wash with dilute hydrochloric acid Adjust the pH to 2-3, and filter with suction to obtain a white solid with a yield of 94.4%. Its NMR data are: 1 H NMR (400MHz, CDCl 3 )δ5.22(t, J=3.2Hz, 1H, 12-CH=C), 4.55-4.43(m, 1H, 3- CH OOCCH 3 ), 2.17(d, J=11.2Hz, 1H, 18-H), 2.04(s, 3H, 2'-CH 3 COO), 2.01-1.80(m, 4H, 11-H+2-H), 1.76-1.58(m, 6H, 16-H+9-H+22-H+20-H), 1.56-1.42(m , 4H, 6a-H+1-H+19-H), 1.37-1.24(m, 5H, 6b-H+21-H+7-H), 1.10-1.02(m, 2H, 15-H), 1.06(s, 3H, 27-CH 3), 0.94 (d, J=8.2Hz, 3H, 30-CH 3 ), 0.93(s, 3H, 23-CH 3 ), 0.86(s, 3H, 25-CH 3 ), 0.85 (d, J=7.5Hz, 3H, 29-CH 3 ), 0.84(s, 3H, 26-CH 3 ), 0.81(s, 1H, 5-H), 0.76(s, 3H, 24-CH 3 ); 13 C NMR (101MHz, CDCl 3 )δ 1...
Embodiment 2
[0057] Embodiment 2: the preparation of intermediate 3β-acetoxy ursolic acid-28-acyl chloride
[0058] Slowly add oxalyl chloride (41.2mmol) dropwise into 30mL of dichloromethane solution dissolved with 3β-acetoxyursolic acid (20.6mmol), stop the reaction after reacting at room temperature for 4h, desolventize the system, and set aside;
Embodiment 3
[0059] Embodiment 3: the preparation of intermediate 3β-acetoxy ursolic acid-28-piperazine
[0060] Dissolve the 3β-acetoxyursolic acid-28-acyl chloride obtained above in 20mL of dichloromethane, and slowly add it dropwise to the solution containing anhydrous piperazine (61.8mmol) and triethylamine (41.2mmol) under ice-bath conditions. ) in 20mL of dichloromethane, reacted at 0°C for 8h, then stopped the reaction, extracted with 50mL of dichloromethane, washed with water, dried, precipitated, and column chromatographed to obtain a white solid with a yield of 86.6%. Its NMR data are: 1 H NMR (400MHz, CDCl 3 )δ5.21 (s, 1H, 12-CH=C), 4.48 (dd, J=8.9, 7.1Hz, 1H, 3- CH OOCCH 3 ), 3.57 (s, 4H, 1'-NCH 2 ), 2.82 (s, 4H, 2'-NCH 2 ), 2.12(d, J=13.2Hz, 1H, 18-H), 2.04(s, 3H, 4'-CH 3 COO), 1.91(d, J=7.2Hz, 2H, 11-H), 1.78-1.57(m, 8H, 2-H+16-H+9-H+22-H+20-H), 1.56- 1.42(m, 4H, 6a-H+1-H+19-H), 1.42-1.22(m, 5H, 6b-H+21-H+7-H), 1.11-1.00(m, 2H, 15- H), 1.06(s, 3H, 27-CH 3 ), 0.93 (d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com