Application of long non coding RNA and interfering RNA thereof to treatment on atherosclerosis
An atherosclerosis, long-chain non-coding technology, applied in the field of biomedicine, can solve problems such as unclear function of lncRNA, and achieve the effect of avoiding the occurrence of clinical time, delaying or even reversing the disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
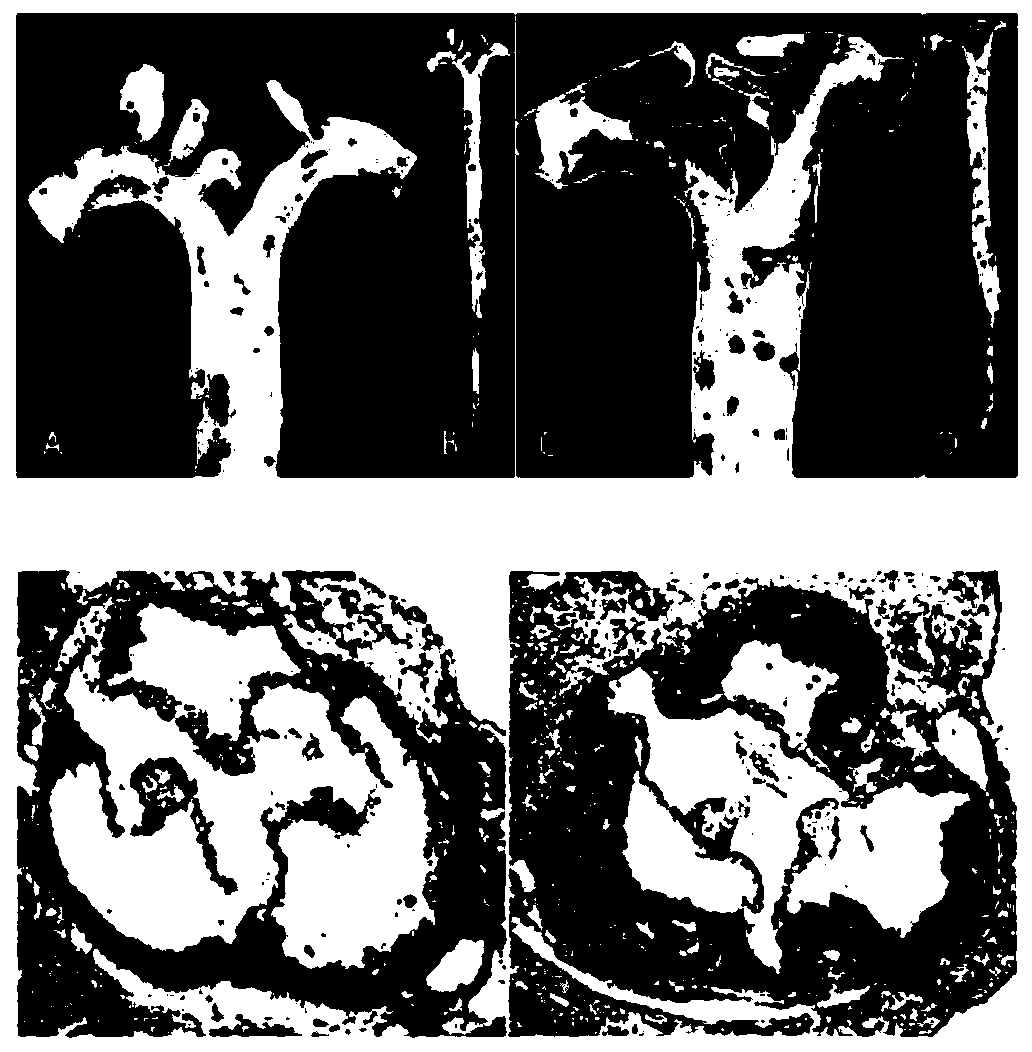
[0026] Example 1: Pathological staining after establishment of atherosclerosis mouse model at different stages
[0027] 1. Materials
[0028] 1.1 Animals
[0029] The Apoe mice of the present invention are purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., and the experimental animals are divided into groups at random, and are respectively fed at the age of 12 weeks (starting high-fat feeding for 4 weeks at the age of 8 weeks, EAR group) and at the age of 24 weeks (8 weeks). At the age of 16 weeks, high-fat feeding was started for 16 weeks, and the aorta was obtained from the ADV group). The use of experimental animals was approved by the Ethics Committee of Harbin Medical University.
[0030] 1.2 Reagents
[0031] High-fat feed (10% lard, 4% milk powder, 2% cholesterol, 0.5% sodium cholate) was purchased from Nanjing Senbao Biological Products Co., Ltd., and the improved oil red O staining solution was purchased from Beijing Suolaibao Co., Ltd. ...
Embodiment 2
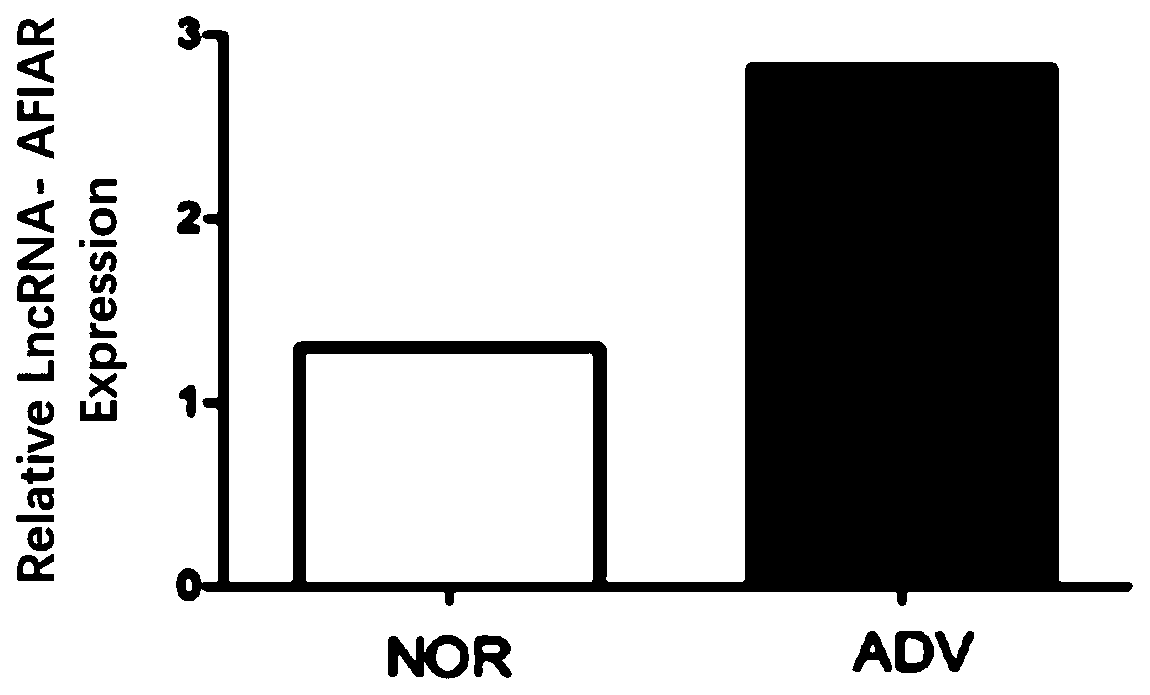
[0041]Example 2: Expression of LncRNA-AFIAR in arterial tissue of mice with atherosclerosis at different stages
[0042] 1. Materials
[0043] 1.1 Organization
[0044] Apoe of the present invention - / - The mice were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The experimental animals were randomly divided into groups, and the main materials were collected at the age of 8 weeks (NOR group) and 24 weeks of age (high-fat feeding for 16 weeks at the age of 8 weeks, ADV group). artery. The use of experimental animals was approved by the Ethics Committee of Harbin Medical University.
[0045] 1.2 Reagents
[0046] TRIzol Reagent was purchased from Invitrogen, USA. The reverse transcription kit (04897030001) was purchased from Roche, Germany. The SYBRGreen Master (ROX) kit used for real-time quantitative PCR (polymerase chain reaction) was purchased from Roche, Germany. Real-Time PCR specific primers were designed and synthesized by Ruibo Xin...
Embodiment 3
[0063] Example 3: Construction of the silencing model of lncRNA-AFIAR in RAW264.7 cells
[0064] 1. Materials
[0065] 1.1 Cells and siRNA
[0066] The RAW264.7 cells used in the present invention are purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences, and the cell culture application contains 10% inactivated fetal bovine serum (Science Cell, USA), penicillin (100 U / mL), streptomycin (100 μg / mL) In DMEM medium, the culture conditions were 37°C, 5% CO2.
[0067] Design related silencing lncRNA-AFIAR (siRNA) sequence, sense strand (Sence): GCAGAUAACUCGUUAGCAAUU (shown in SEQ ID NO.4), antisense strand (Antisense): UUGCUAACGAGUUAUCUGCUU (shown in SEQ ID NO.5). The design and synthesis of small interfering RNA (siRNA) were completed by Suzhou Gemma Gene Co., Ltd.
[0068] 1.2 Reagents
[0069] X-tremeGENE siRNA Transfection Reagent used for small interfering RNA (siRNA) transfection was purchased from Roche Company in Germany; DMEM medium was purchased fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com