Blood serum/blood plasma miRNA marker related to non-small cell lung cancer (SCLC) prognosis and application thereof
A non-small cell lung cancer and marker technology, applied in DNA/RNA fragments, recombinant DNA technology, microbial measurement/inspection, etc., can solve the problem of poor prognosis of non-small cell lung cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] The collection of embodiment 1 sample and the arrangement of sample data
[0095] The inventor has collected a large number of serum samples from NSCLC patients from Jiangsu Cancer Hospital and the First Affiliated Hospital of Nanjing Medical University since July 2003. After sorting out the sample data, the inventor selected 303 samples that meet the following criteria Experimental samples for Solexa sequencing and subsequent series of qRT-PCR verification:
[0096] 1. New stage I-IIIa adenocarcinoma and squamous cell carcinoma;
[0097] 2. All underwent surgery and postoperative adjuvant chemotherapy;
[0098] 3. No surgery, radiotherapy and chemotherapy before blood collection, no preoperative radiotherapy and chemotherapy.
[0099] The demographic data, clinical data and follow-up data of these samples were collected systematically.
Embodiment 2
[0100] The Solexa sequencing experiment of microRNA in embodiment 2 serum / plasma
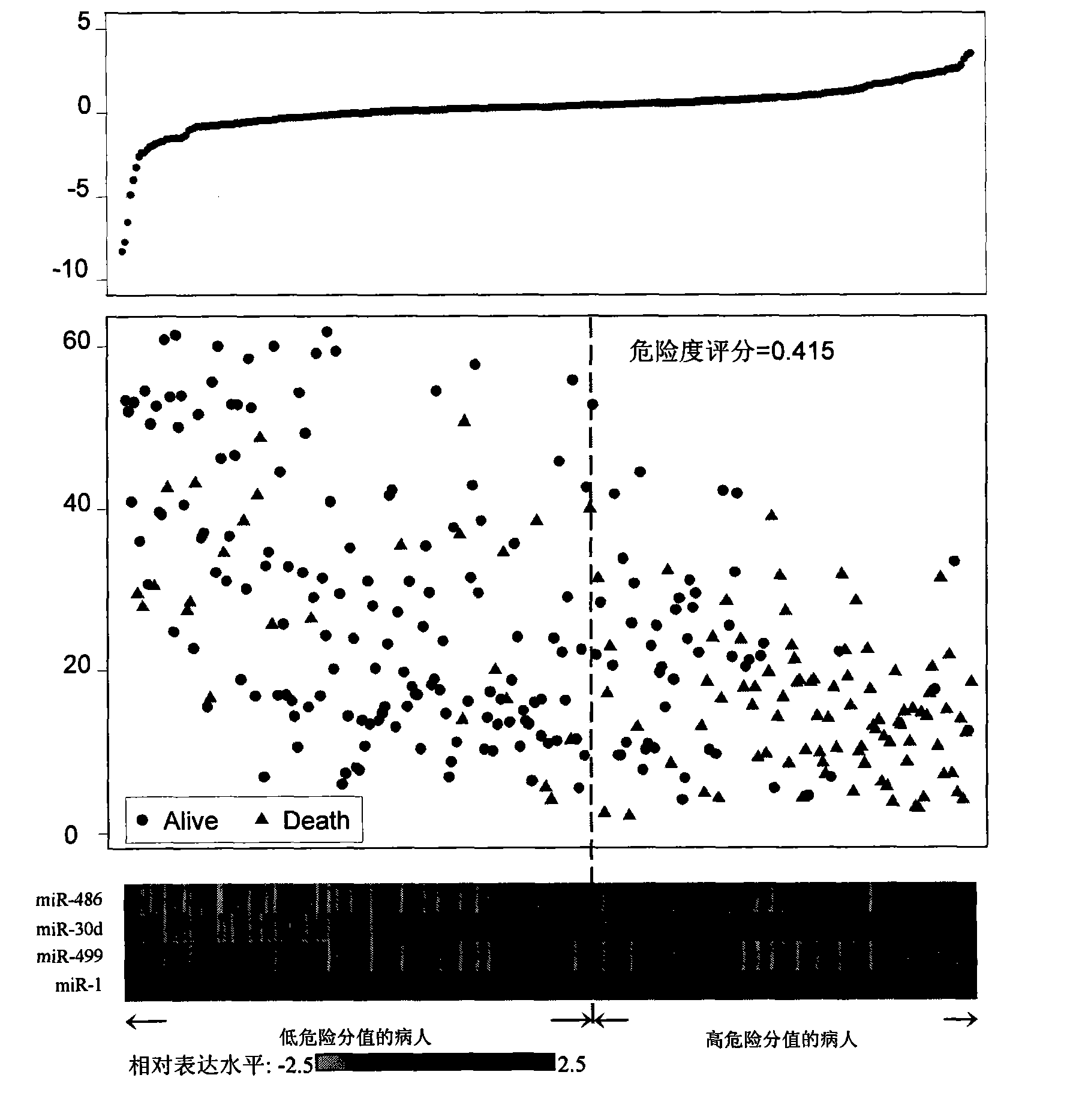
[0101] Among the above 303 eligible NSCLC patients, 30 surviving patients with a survival period of more than 30 months (34.6-61.8 months, average 49.54 months) at the last follow-up were selected as the "long-term survival" group, and another 30 patients were selected Dead patients with a survival time of less than 25 months (2.0-22.5 months, average 9.54 months) were taken as the "short-term survival" group. The clinical stage of the disease and smoking status were accurately matched between the two groups, and both underwent surgery and postoperative adjuvant chemotherapy. There was no difference in tissue types, and they were basically evenly comparable except for survival conditions and survival time (see Table 5). These two groups of people were used as exploratory samples to obtain related results through Solexa sequencing test. The specific steps are:
[0102] 1. Take 50ml of serum fro...
Embodiment 3
[0114] The qRT-PCR experiment of microRNA in embodiment 3 exploratory sample serum
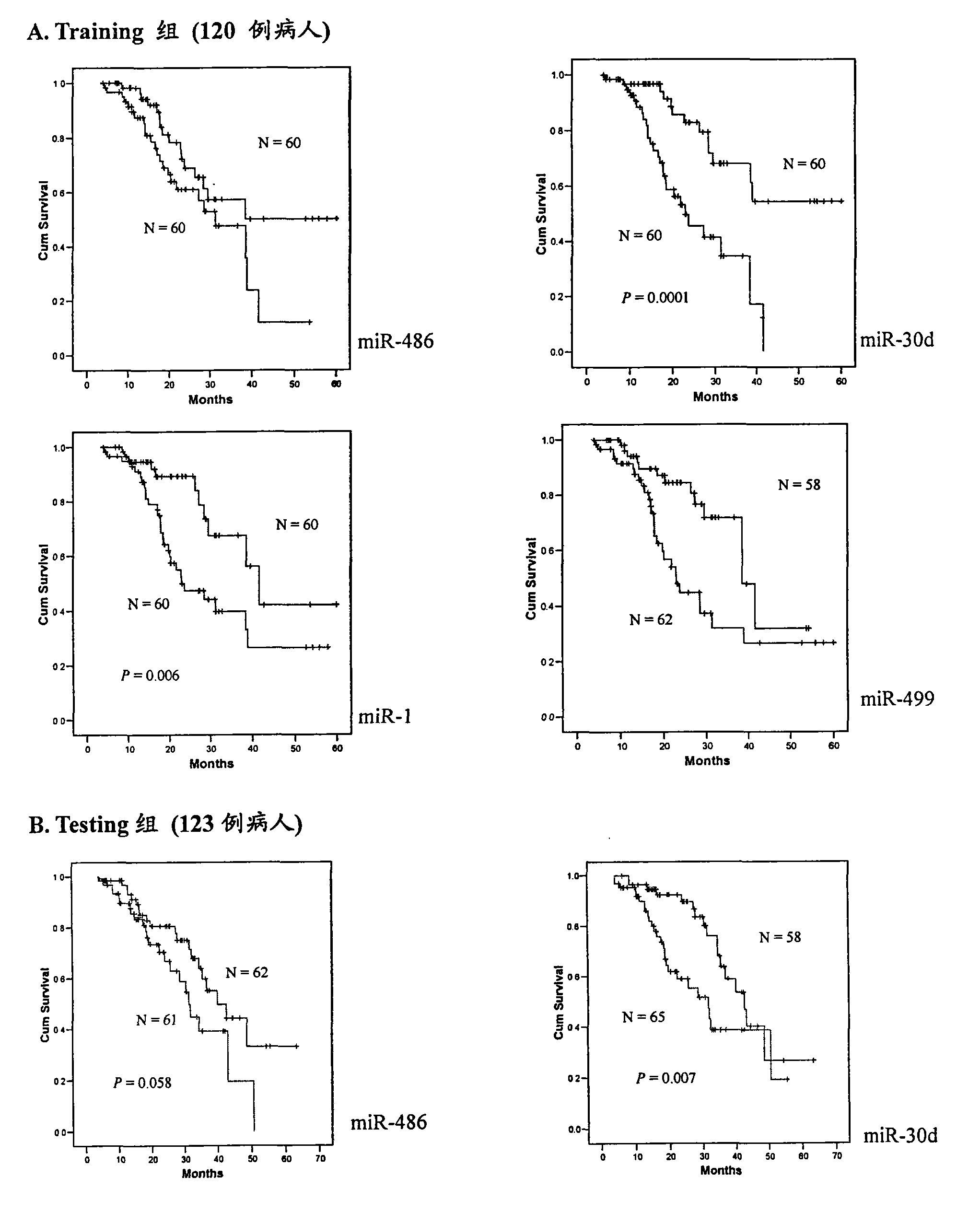
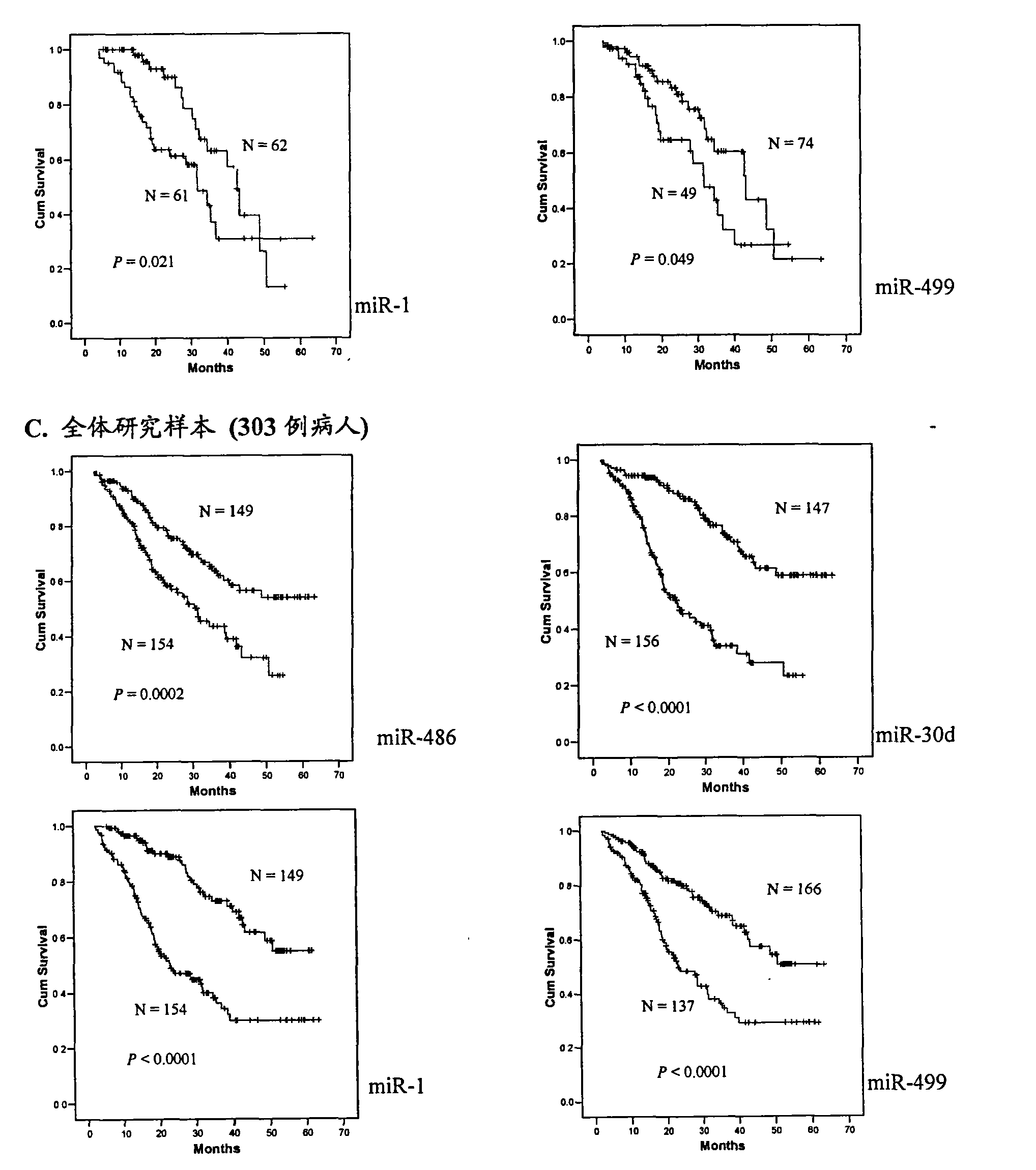
[0115] According to the above-mentioned Solexa results, miRNAs that meet the following conditions are selected for further verification by qRT-PCR method: 1) miRNAs with a fold difference of up to 5 times in the two groups of NSCLC patients are used as serum biomarkers for preliminary screening in the present invention, 2) The copy number of these miRNAs in at least one group of NSCLC patients ("long-term survival" group or "short-term survival" group) is greater than 50 to improve detection efficiency. miRNAs meeting the above conditions include: miR-486, miR-22, miR-30d, miR-21, miR-26b, let7i, miR-378, miR-1, miR-206, miR-146b and miR-499. In addition, two let-7 family members (let-7a and let-7g) have only a two-fold difference, but these two miRNAs have been reported to be associated with lung cancer prognosis, so they were also included in our study. Primers for reverse transcription and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com