Synthesis method of nucleoside compound and intermediate of nucleoside compound
A nucleoside and ribose technology, applied in the field of chemistry, can solve the problems of unfavorable large-scale synthesis, low X1 yield and high synthesis cost, and achieve the effects of improving reaction yield, easy solubility and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
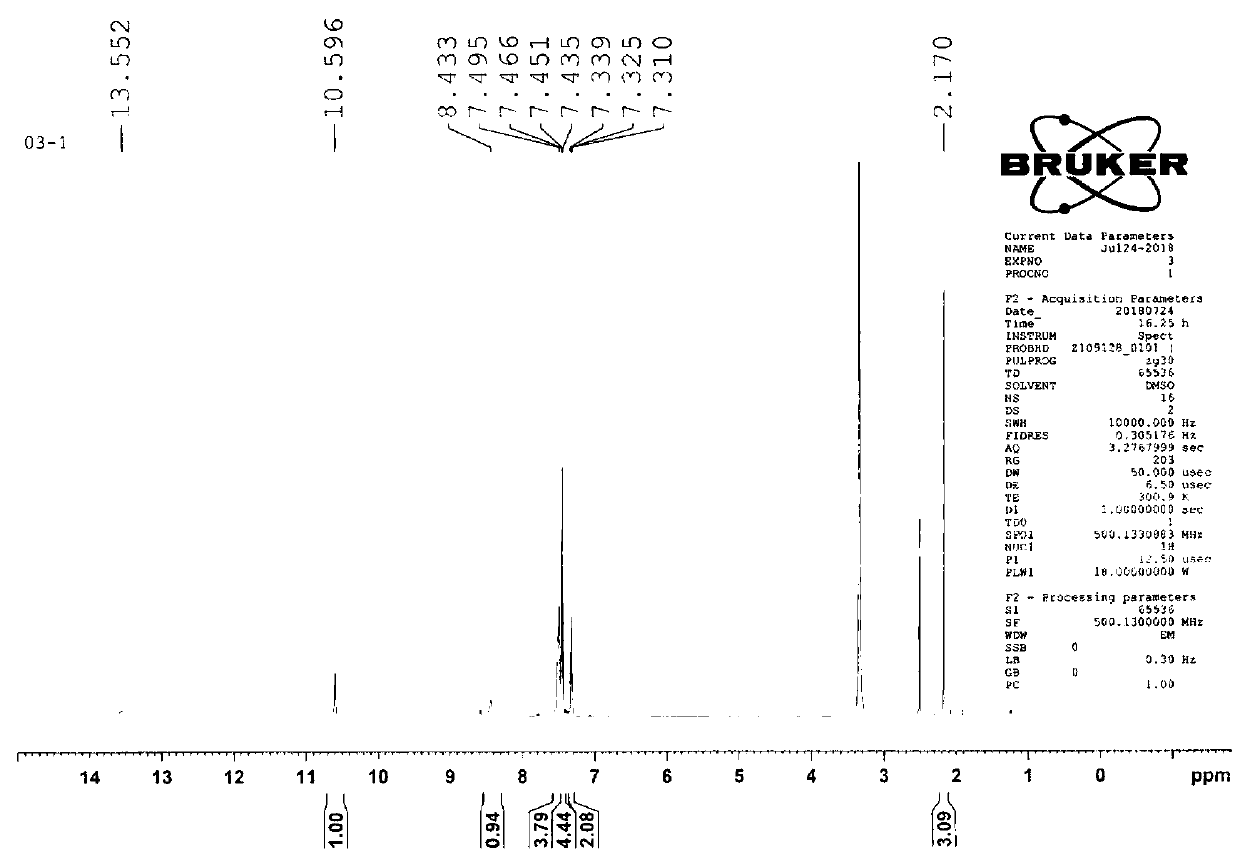
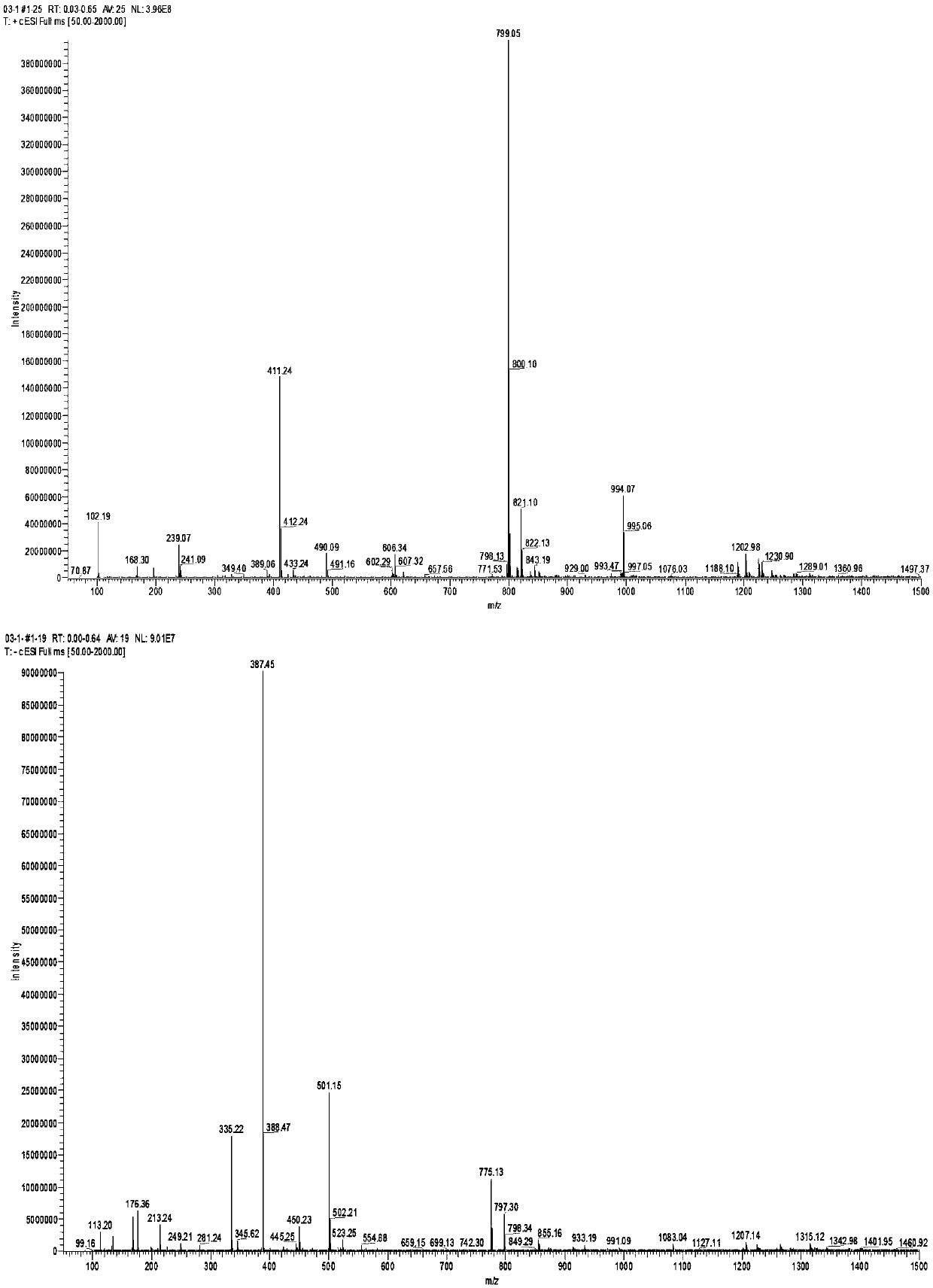
[0072] Step 1, the synthesis of X6 (2,9-diacetylguanine)
[0073] Dissolve 10gX5 (guanine) in 85mL N,N-dimethylacetamide, then add 20mL acetic anhydride Ac 2 O, under the protection of nitrogen, the temperature was raised to 165°C for 17h. After the reaction of the raw materials was complete, the reaction solution was cooled to room temperature and allowed to stand for 12 hours to crystallize, and a large amount of solids precipitated out. The product X6 (2,9-diacetylguanine) was filtered and dried to obtain 14.3 g, with a yield of 92.1%.
[0074] 1 HNMR (500MHz, DMSO-d6) δ: 2.19 (s, 3H), 2.80 (s, 3H), 8.30 (s, 1H), 11.68 (br s, 2H); 13CNMR (DMSO-d6, 75.5MHz) δ: 23.9, 24.7, 121.7, 137.7, 147.3, 154.9, 168.3, 173.6, 174.0; MS (ESI), m / z: 235.20 ([M+H]+).
[0075] Wherein, X5 (guanine) structural formula is:
[0076]
[0077] The structural formula of X6 (2,9-diacetylguanine) is:
[0078]
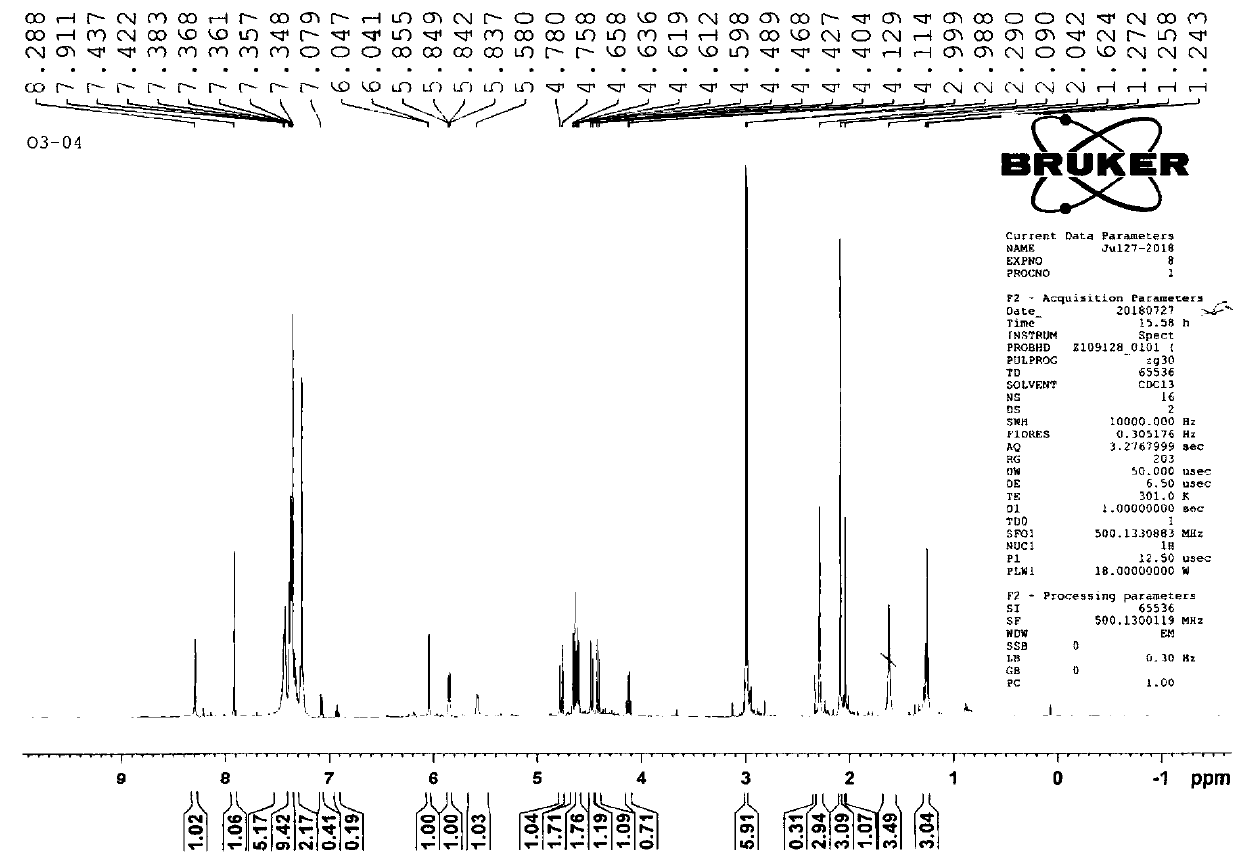
[0079] Step 2, the synthesis of X7 (2-acetyl-6-oxodibenzamide-guanine)
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com