Application of dehydronitrosonisoldipine in preparing of drugs for sepsis prevention and treatment
A technology of nitrosinoxone and sepsis, applied in the field of medicine, can solve the problem that the effect of inhibiting pyroptosis has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
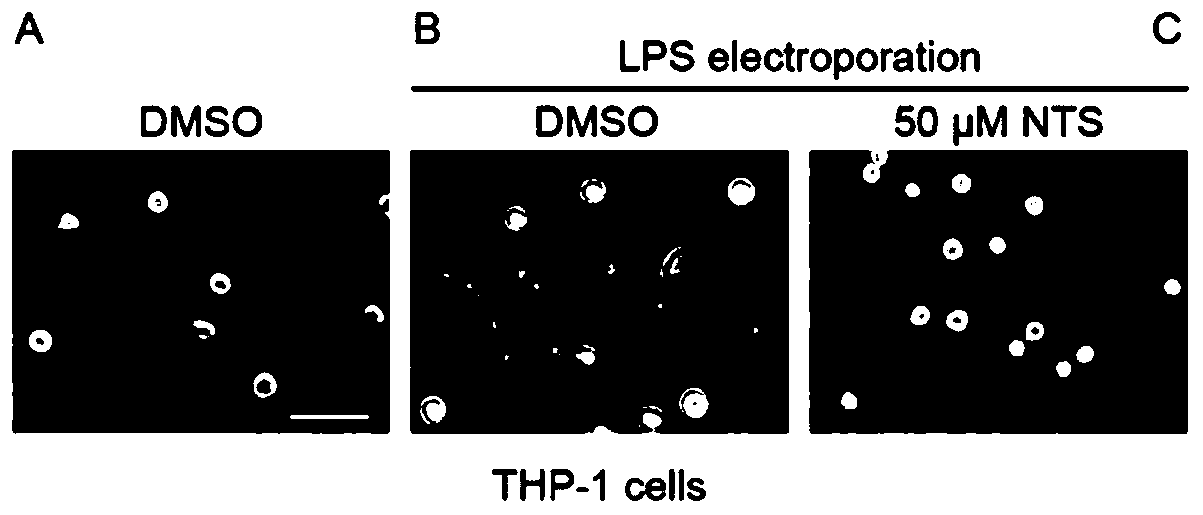
[0024] Example 1: NTS inhibits endotoxin-induced pyroptosis of human monocyte cell line THP-1.
[0025] Experimental materials: NTS (MedChemExpress, Cat. No. HY-Z0816) was dissolved in dimethylsulfoxide (DMSO). Endotoxin Escherichia coli O111:B4 LPS (Sigma, Cat. No. LPS25) was dissolved using sterile water. The THP-1 cell line was provided by the Shanghai Institute of Cell Biology, Chinese Academy of Sciences, and was cultured using RMPI 1640 culture medium supplemented with 10% fetal bovine serum and 1% penicillin / streptomycin. The Neon electroporator was purchased from Invitrogen. The model of optical microscope is Olympus IX71.
[0026] Experimental method: We added 50 μM NTS or an equal volume of DMSO to THP-1 cell culture medium and shaken; after 30 minutes, according to the method described in the literature (Zhao Y, Shi J, Shao F. Inflammatory Caspases: Activation and Cleavage of Gasdermin-D In Vitro and During Pyroptosis[J].Methods Mol Biol,2018,1714:131-148), using...
Embodiment 2
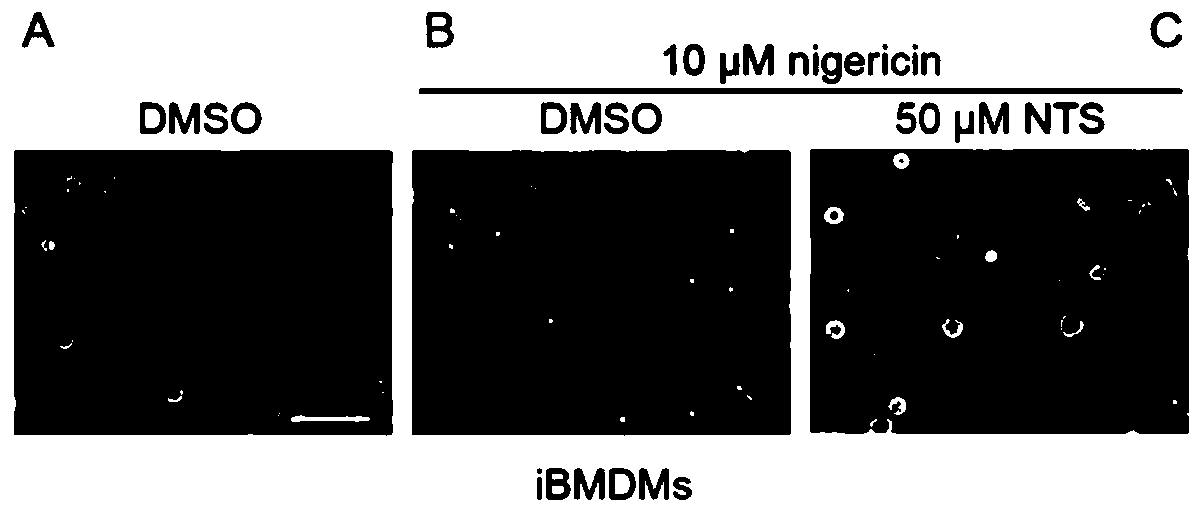
[0028] Example 2: NTS inhibits Nigerian toxin-induced pyroptosis of iBMDM cells in a mouse macrophage cell line.
[0029] Experimental materials: NTS (MedChemExpress, Cat. No. HY-Z0816), nigericin (Sigma, Cat. No. N7143) were dissolved in DMSO. The immortalized mouse-derived macrophage cell line (immortalized bonemarrow derived macrophage, iBMDM) was generously provided by Academician Shao Feng of the Beijing Institute of Life Sciences. It was cultured in RMPI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin / streptomycin. The model of optical microscope is Olympus IX71.
[0030] Experimental method: We added 50 μM NTS or an equal volume of DMSO to the cell culture medium of iBMDM and shaken; 30 minutes later, 10 μM nigericin was added to the culture medium to induce cell pyroptosis. After 1 hour, we observed and photographed cell morphology using a light microscope.
[0031] Experimental results: see attached figure 2 , panel A shows the negative con...
Embodiment 3
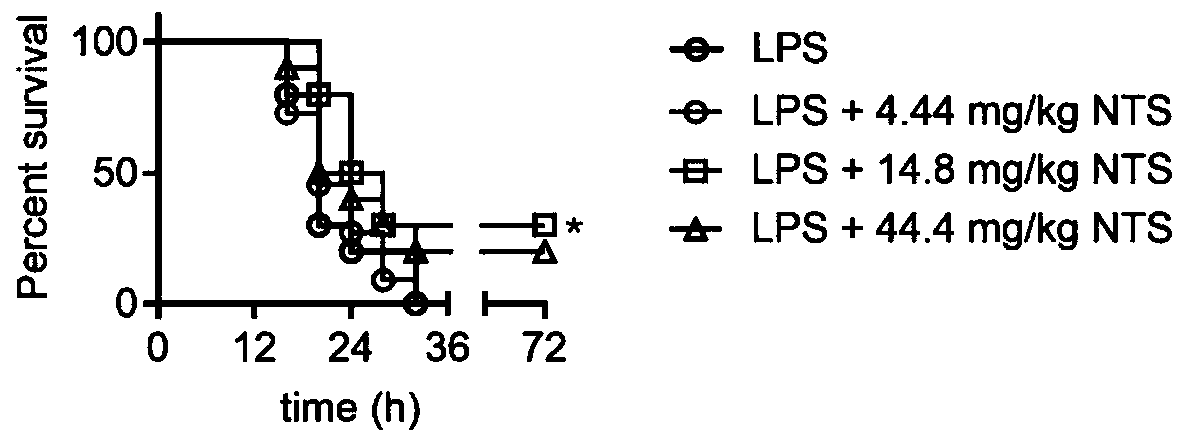
[0032] Example 3: NTS improves the survival rate of LPS sepsis mice and inhibits the expression of inflammatory factors
[0033] Experimental material: NTS (MedChemExpress, Cat.No.HY-Z0816) was dissolved in DMSO. Endotoxin Escherichia coli O111:B4 LPS (Sigma, Cat. No. LPS25) was dissolved using sterile water. The mice are C57 / BL6 strain, male, aged 6-8 weeks, weighing about 25 g, provided by the Model Animal Research Center (MARC) of Nanjing University. Mice were kept in a specific pathogen free (SPF) environment certified by the International Laboratory Animal Evaluation and Accreditation Committee, and all experiments were approved by the MARC Animal Care and Use Committee. The levels of interleukin (interleukin, IL)-1β, IL-6 and tumor necrosis factor-α (tumor necrosis factor-α, TNF-α) were detected by ELISA kits (Cat.No.MLB00C, Cat.No.M6000B, and Cat.No.M6000B, and Cat. .No.MTA00B, R&D Systems).
[0034] Experimental method: NTS was formulated with DMSO at a concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com