O-alkenyl aromatic nitrile compound and preparation method thereof
A compound and aromatic nitrile technology, applied in the field of o-alkenyl aromatic nitrile compounds and their preparation, can solve the problems of high cost and complex synthesis method, and achieve the effects of simple and efficient synthesis, good atom economy and step economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The application discloses that the preparation method of o-alkenyl aromatic nitrile compound preferably comprises the following steps: under an atmosphere of atmospheric pressure oxygen, add imidate derivatives (0.1 mmol) shown in formula (II) successively in the reactor, Then add 2.5 mg of dichloro(pentamethylcyclopentadienyl) rhodium dimer, 7.8 mg of bistrifluoromethanesulfonimide silver salt, 4.9 mg of sodium acetate and 12.0 mg of copper acetate, injecting (III) A solution of the alkene compound (0.2 mmol) in 1,2-dichloroethane (1.0 mL) was placed in a reactor at 100° C. for 12 h, The end of the reaction was confirmed by thin-layer chromatography analysis. After the reaction solution was filtered through diatomaceous earth, it was concentrated by rotary evaporation with 400 mesh silica gel to make a dry powder, and then the reaction product was separated by column chromatography. The 400 mesh silica gel was 10 grams, and the developer was volume Petroleum ether an...
Embodiment 1
[0064] The present embodiment carries out the preparation of (E)-2-(3-bromostyryl)-benzonitrile (1a), and its reaction formula is as follows:
[0065]
[0066] Under an atmosphere of oxygen at atmospheric pressure, the formula is added successively in the reactor to add 2.5mg dichloro(pentamethylcyclopentadienyl) rhodium dimer, 7.8mg bistrifluoromethanesulfonylimide silver salt, 4.9 mg sodium acetate and 12.0 mg copper acetate, followed by adding imidate derivative 2a (14.9 mg, 0.1 mmol) shown in formula (II), and injecting olefin compound 3a (36.2 mg, 0.2 mmol) shown in formula (III) with a syringe ) solution of 1,2-dichloroethane (1.0mL) in the reactor and placed at 100°C for 12h, the end of the reaction was confirmed by thin-layer chromatography analysis, the reaction solution was filtered through diatomaceous earth and filtered through 400 mesh The silica gel is concentrated by rotary evaporation to make a dry powder, and then the reaction product is separated by column...
Embodiment 2
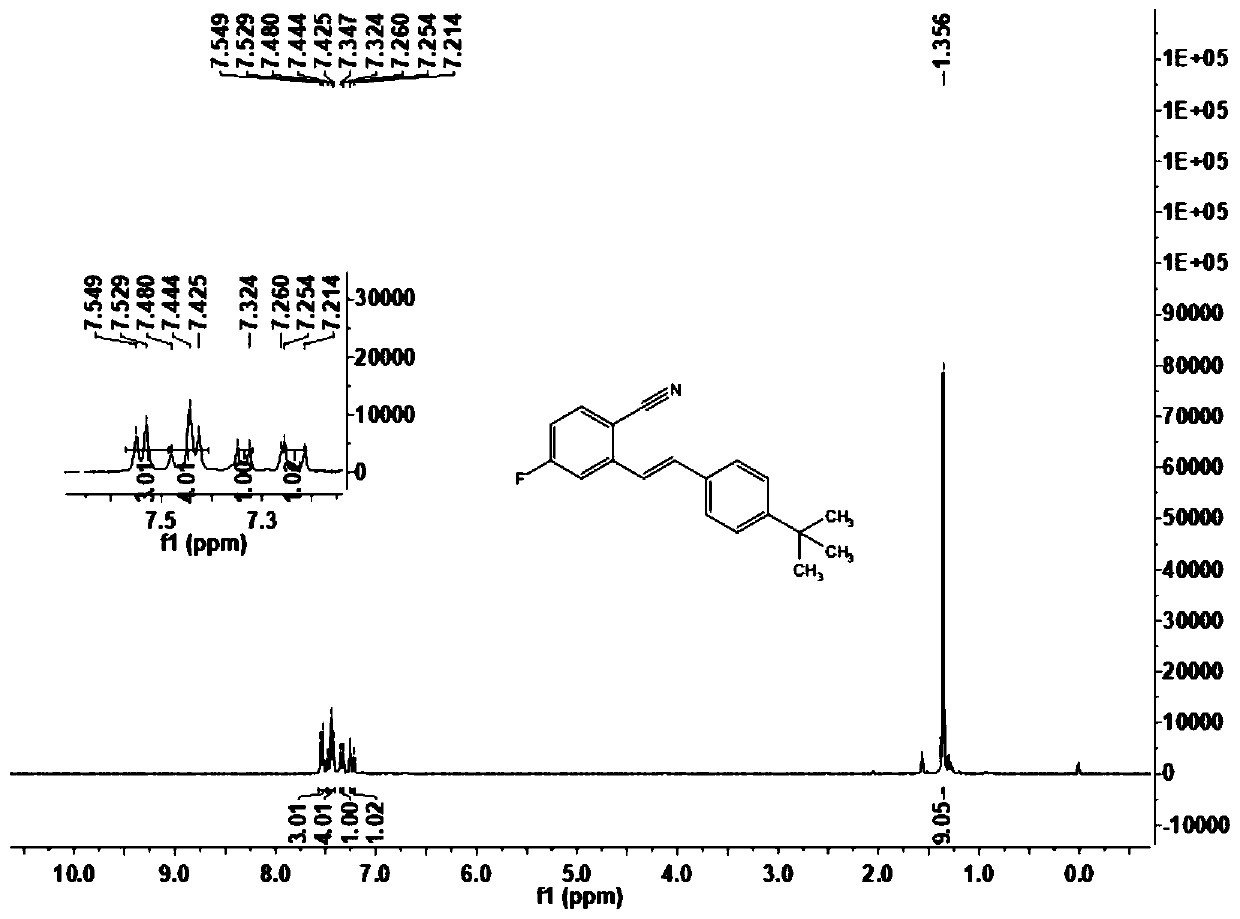
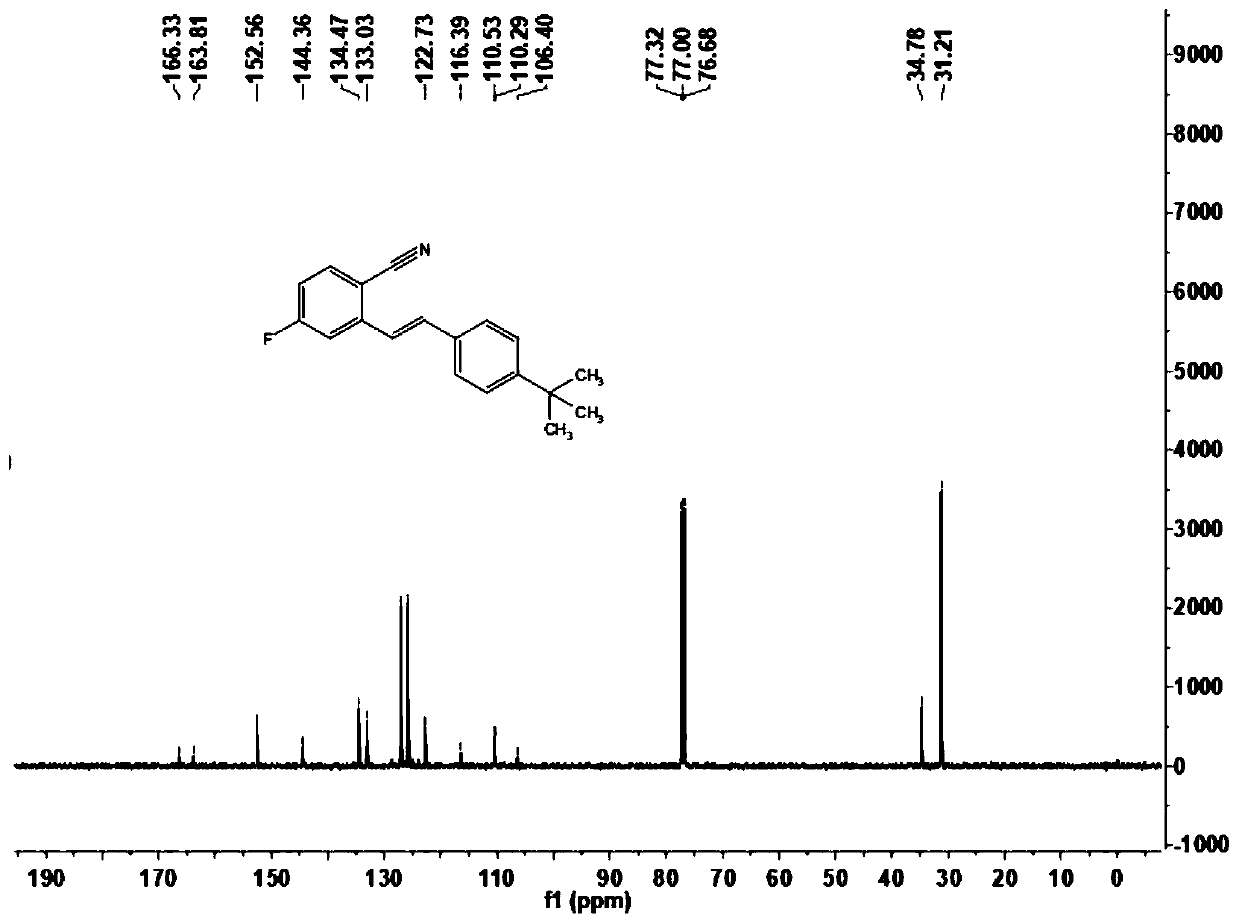
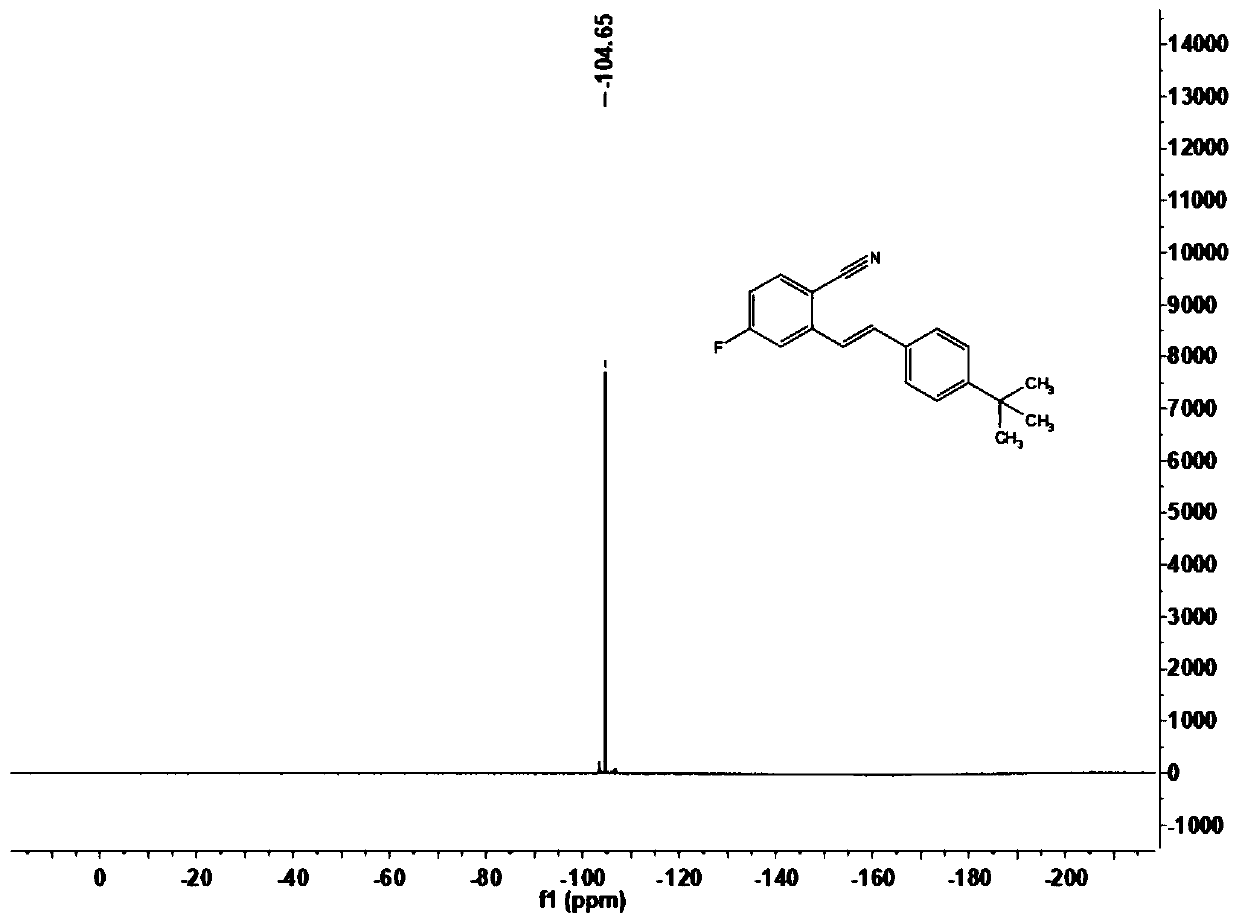
[0070] This embodiment carries out the preparation of (E)-2-(4-tert-butylstyryl)-4-fluorobenzonitrile (1b), and its reaction formula is as follows:
[0071]
[0072] Under an atmosphere of oxygen at atmospheric pressure, the formula is added successively in the reactor to add 2.5mg dichloro(pentamethylcyclopentadienyl) rhodium dimer, 7.8mg bistrifluoromethanesulfonylimide silver salt, 4.9 mg sodium acetate and 12.0 mg copper acetate, then add the imidate derivative 2b (16.7mg, 0.1mmol) shown in formula (II), and inject the olefin compound 3b (32.0mg, 0.2mmol) shown in formula (III) with a syringe ) solution of 1,2-dichloroethane (1.0mL) in the reactor and placed at 100°C for 12h, the end of the reaction was confirmed by thin-layer chromatography analysis, the reaction solution was filtered through diatomaceous earth and filtered through 400 mesh The silica gel is concentrated by rotary evaporation to make a dry powder, and then the reaction product is separated by column ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com