Acetylenic ketone derivative as well as preparation method and application thereof
A technology of derivatives and ketones, which is applied in the field of ketone derivatives and its preparation, can solve problems such as danger and inconvenient use, and achieve the effect of rich reactivity, simple and efficient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
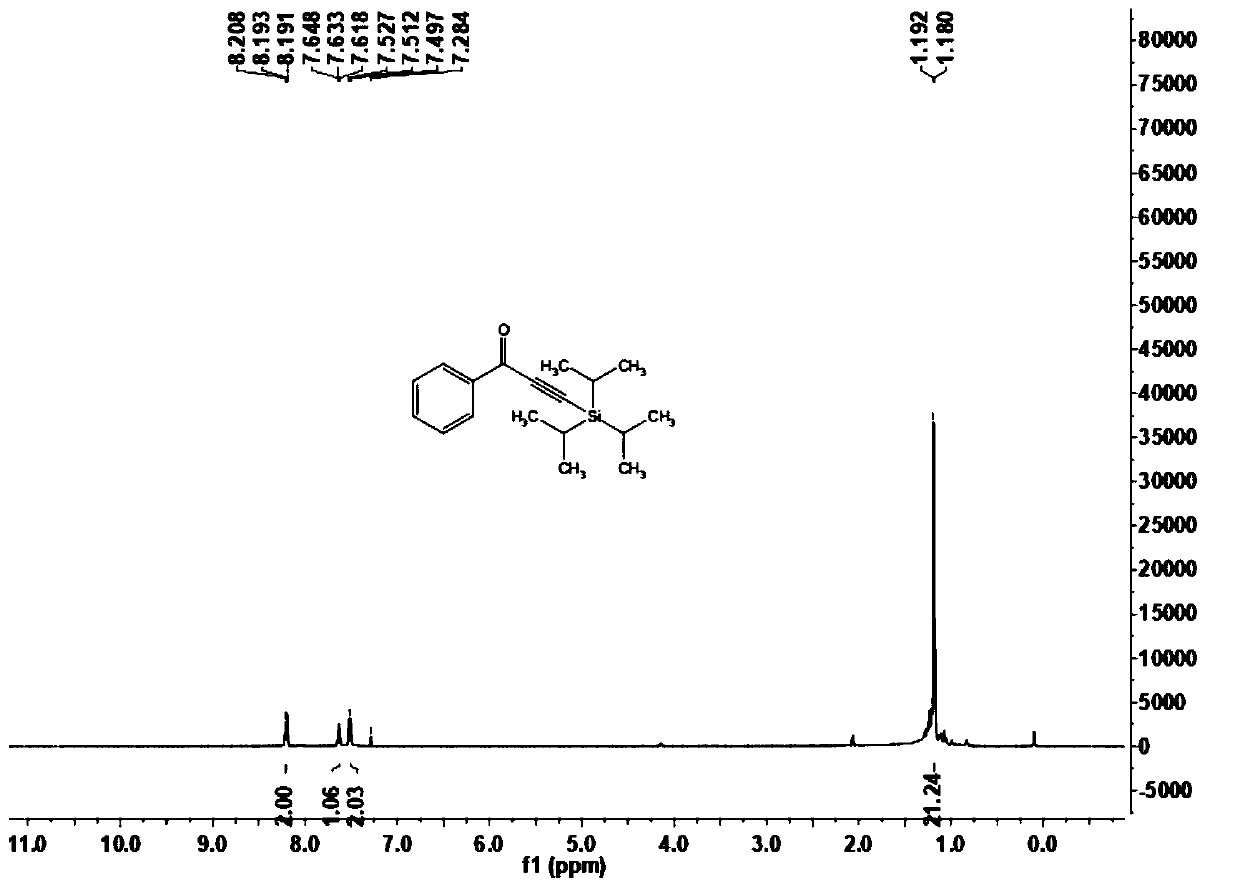
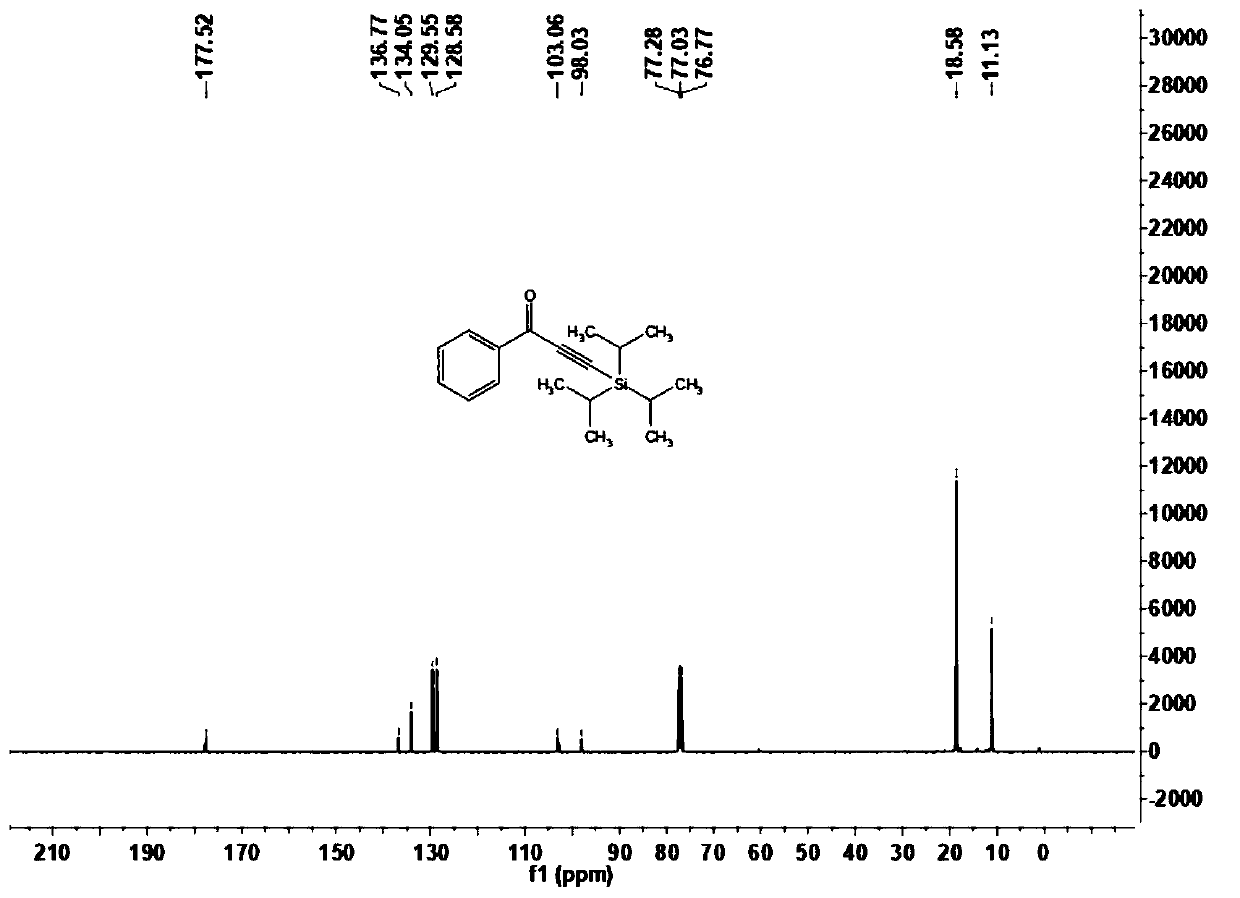
[0068] This embodiment carries out the preparation of 1-phenyl-3-(triisopropylsilyl)prop-2-yn-1-one (1a), and its reaction formula is as follows:
[0069]
[0070] Under a nitrogen atmosphere, 6.1 mg of ethylene glycol dimethyl ether nickel bromide, 4.7 mg of terpyridine, 5.8 mg of zinc chloride and 12.8 mg of zinc powder were successively added to the reactor, and the carboxylic acid shown in formula (II) was injected with a syringe. Thioester (0.2mmol), the solution of the mixed solvent (0.2mL / 0.2mL) of N,N-dimethylacetamide and tetrahydrofuran (0.2mL / 0.2mL) of alkynylation reagent (0.3mmol) shown in formula (III) is placed in the reactor React at room temperature for 24 hours, and the end of the reaction was confirmed by thin-layer chromatography analysis. The reaction solution was filtered through diatomaceous earth and then concentrated into dry powder with 400 mesh silica gel by rotary evaporation, and then the reaction product was separated by column chromatography. 4...
Embodiment 2
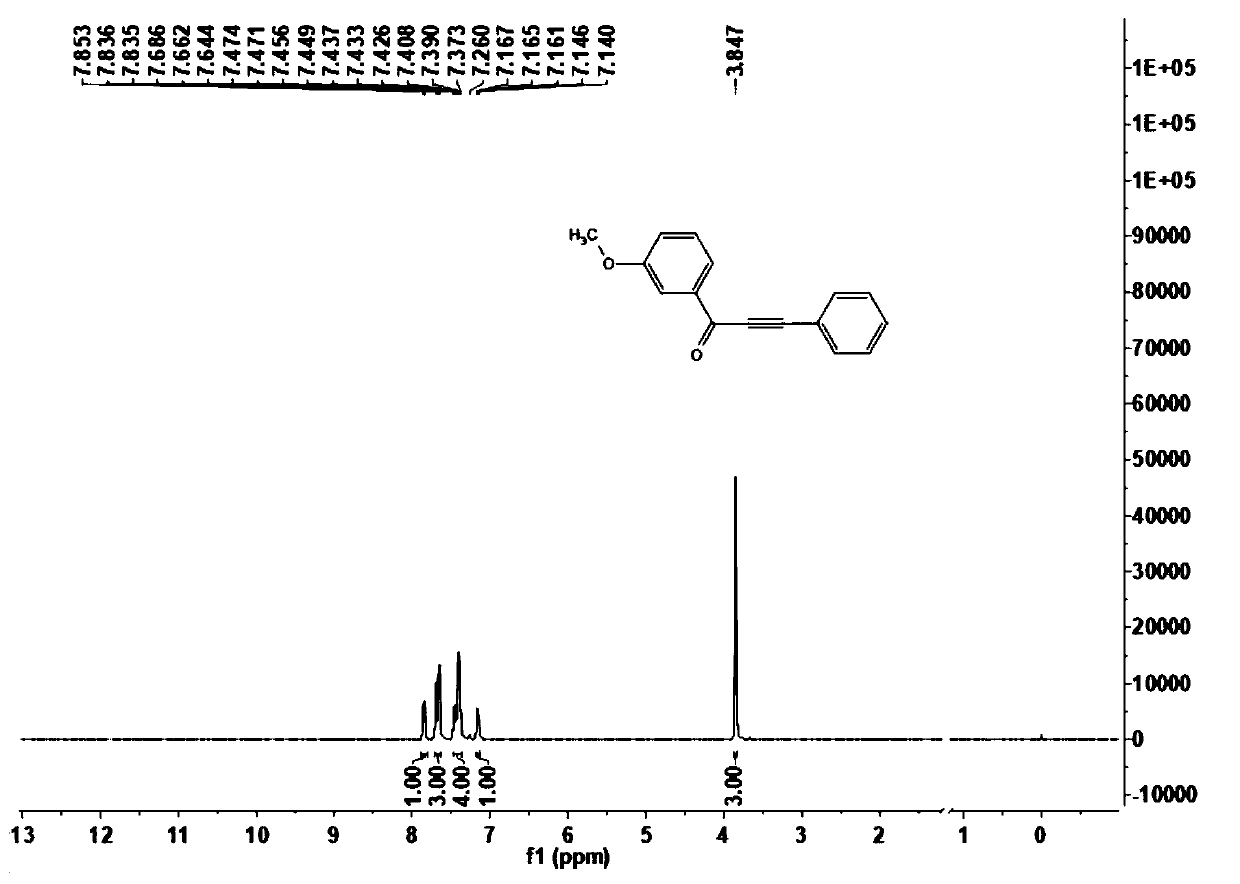
[0074] This embodiment carries out the preparation of 1-(3-methoxyphenyl)-3-phenylpropyl-2-yn-1-one (1b), and its reaction formula is as follows:
[0075]
[0076] Under a nitrogen atmosphere, 6.1 mg of ethylene glycol dimethyl ether nickel bromide, 4.7 mg of terpyridine, 5.8 mg of zinc chloride and 12.8 mg of zinc powder were successively added to the reactor, and the carboxylic acid shown in formula (II) was injected with a syringe. Thioester (0.2mmol), the solution of the mixed solvent (0.2mL / 0.2mL) of N,N-dimethylacetamide and tetrahydrofuran (0.2mL / 0.2mL) of alkynylation reagent (0.3mmol) shown in formula (III) is placed in the reactor React at room temperature for 24 hours, and the end of the reaction was confirmed by thin-layer chromatography analysis. The reaction solution was filtered through diatomaceous earth and then concentrated into dry powder with 400 mesh silica gel by rotary evaporation, and then the reaction product was separated by column chromatography. 4...
Embodiment 3
[0080] This embodiment carries out the preparation of 3-phenyl-1-(thiophen-2-yl)prop-2-yn-1-one (1c), and its reaction formula is as follows:
[0081]
[0082] Under a nitrogen atmosphere, 6.1 mg of ethylene glycol dimethyl ether nickel bromide, 4.7 mg of terpyridine, 5.8 mg of zinc chloride and 12.8 mg of zinc powder were successively added to the reactor, and the carboxylic acid shown in formula (II) was injected with a syringe. Thioester (0.2mmol), the solution of the mixed solvent (0.2mL / 0.2mL) of N,N-dimethylacetamide and tetrahydrofuran (0.2mL / 0.2mL) of alkynylation reagent (0.3mmol) shown in formula (III) is placed in the reactor React at room temperature for 24 hours, and the end of the reaction was confirmed by thin-layer chromatography analysis. The reaction solution was filtered through diatomaceous earth and then concentrated into dry powder with 400 mesh silica gel by rotary evaporation, and then the reaction product was separated by column chromatography. 400 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com