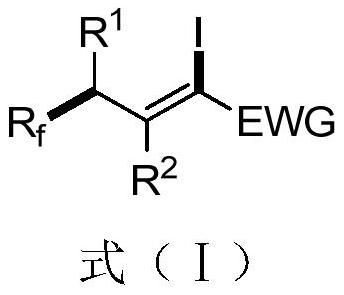
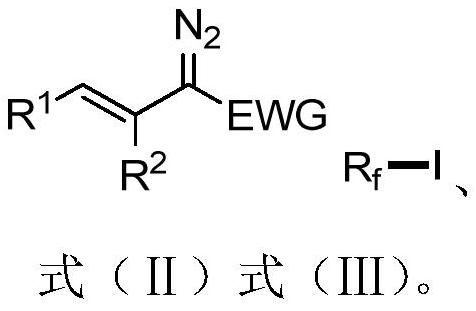
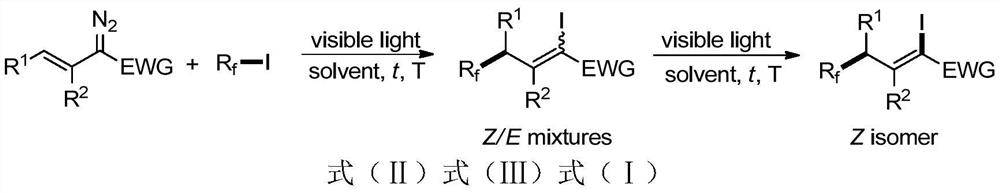
A 1-iodo-3-perfluoroalkyl olefin compound and its preparation method
A technology of perfluoroalkyl olefins and perfluoroalkyl iodides, applied in the field of 1-iodo-3-perfluoroalkyl olefin compounds and their preparation, to achieve high yield, high cis-trans selectivity, and rich reactivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 (Z)-5,5,5-trifluoro-2-iodopent-2-enoic acid butyl ester
[0050]
[0051] Add 0.4mmol n-butyl alkenyldiazoacetate, an appropriate amount of acetonitrile and 0.8mmol trifluoroiodomethane into a Schlenk bottle, and react under 18W CFL light irradiation for 1h at room temperature. Subsequently, the reaction solution was concentrated to remove excess trifluoroiodomethane, and then an appropriate amount of acetonitrile and 0.06 mmol of dimanganese decacarbonyl catalyst were added to the reaction test tube. After continuing to react for 9 hours under the same light source, the reaction solution was concentrated, petroleum ether / Ethyl acetate = 50:1 mixed solvent as eluent column chromatography purification to obtain (Z)-5,5,5-trifluoro-2-iodopent-2-enoic acid butyl ester with a yield of 85% , Z / E>30:1. Its structure is as follows:
[0052]
[0053] The NMR and high-resolution data of the compound are as follows:
[0054] 1 H NMR (400MHz, CDCl 3 )δ7.26(t,...
Embodiment 2
[0058] Example 2 (Z)-5,5,6,6,7,7-heptafluoro-2-iodohept-2-enoic acid butyl ester
[0059]
[0060] Add 0.4mmol n-butyl alkenyl diazoacetate, an appropriate amount of acetonitrile and 1.6mmol perfluoroiodopropane into a Schlenk bottle, and react under sunlight irradiation for 5h at room temperature. Subsequently, the reaction solution was concentrated to remove excess heptafluoroiodopropane, and then an appropriate amount of acetonitrile and 0.01 mmol of dimanganese decacarbonyl catalyst were added to the reaction test tube, and the reaction was continued for 1 h under the same light source, and the reaction solution was concentrated, petroleum ether / Ethyl acetate = 50:1 mixed solvent as eluent column chromatography to obtain (Z)-5,5,6,6,7,7-heptafluoro-2-iodohept-2-enoic acid butyl ester , the yield is 86%, Z / E>30:1. Its structure is as follows:
[0061]
[0062] The NMR and high-resolution data of the compound are as follows:
[0063] 1 H NMR (400MHz, CDCl 3 )δ7.3...
Embodiment 3
[0067] Example 3 (Z)-5,5,6,6,7,7,8,8,8-nonafluoro-2-iodo-2-enoic acid butyl ester
[0068]
[0069] Add 0.4 mmol n-butyl alkenyl diazoacetate, an appropriate amount of dichloromethane and 2.0 mmol nonafluoro-n-butyl iodide into a Schlenk bottle, and react under 5W LED white light irradiation for 10 h at room temperature. Subsequently, the reaction solution was concentrated to remove excess nonafluoro-n-butyl iodide, and then an appropriate amount of dichloromethane and 0.15 mmol of dimanganese decacarbonyl catalyst were added to the reaction test tube, and the reaction was continued for 12 hours under the same light source, and the reaction solution was concentrated , petroleum ether / ethyl acetate=50:1 mixed solvent as the eluent column chromatography to obtain (Z)-5,5,6,6,7,7,8,8,8-nonafluoro-2 - Iodo-2-enoic acid butyl ester, the yield is 85%, Z / E>30:1. Its structure is as follows:
[0070]
[0071] The NMR and high-resolution data of the compound are as follows:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com