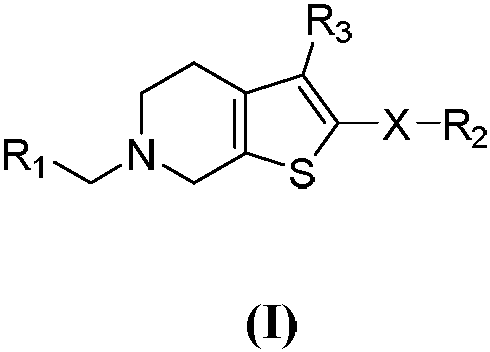
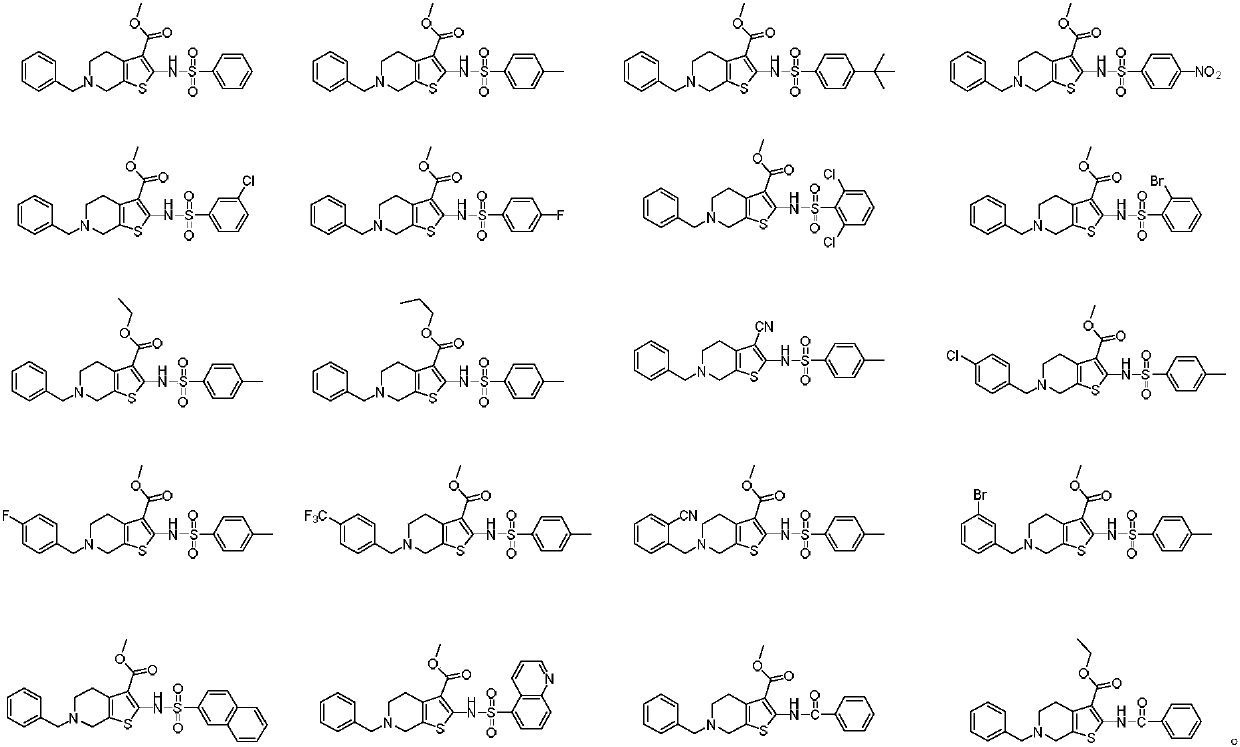
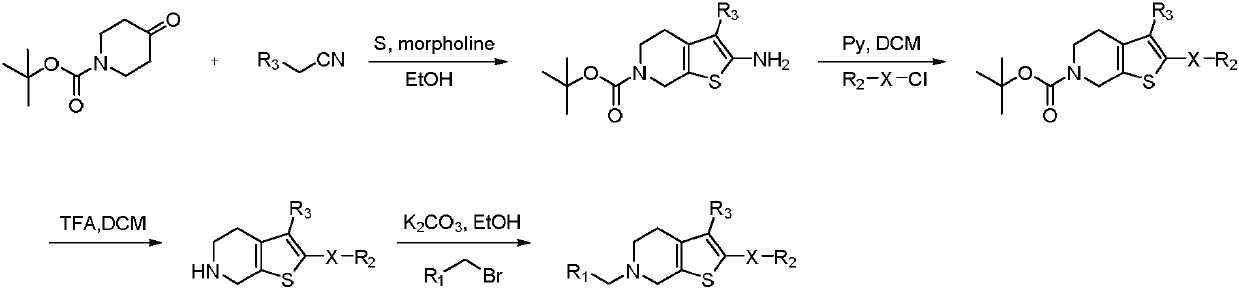
Selective butyrylcholine esterase inhibitor, preparation method and uses thereof
A butyrylcholinesterase and selective technology, which is applied in the direction of pharmaceutical formulations, organic active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of no drugs available for patients and the lack of drugs for patients with severe AD, and achieve Good in vitro activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) 6-(tert-butyl) 3-methyl 2-amino-4,7-dihydrothieno[2,3-c]pyridine-3,6(5H)-dicarboxylate (intermediate 1 )Synthesis
[0050] Take N-tert-butoxycarbonyl-4-piperidone (2g, 10.4mmol) in an eggplant-shaped bottle, dissolve it with ethanol (20mL), add methyl cyanoacetate (1.09g, 11.04mmol), and settle sulfur (0.39g , 12.05mmol) and morpholine (1.75g, 20.08mmol), heated to reflux for 3 hours, cooled to room temperature and stirred overnight, a large amount of solids were precipitated, filtered, the filter cake was washed twice with ice ethanol, dried to obtain intermediate 6- (tert-butyl) 3-methyl 2-amino-4,7-dihydrothieno[2,3-c]pyridine-3,6(5H)-dicarboxylate (2.42g, yield 77% ).
[0051] (2) 6-(tert-butyl)3-methyl 2-(benzenesulfonamido)-4,7-dihydrothieno[2,3-c]pyridine-3,6(5H)-dicarboxylic acid Synthesis of Esters (Intermediate 2)
[0052] Take 6-(tert-butyl) 3-methyl 2-amino-4,7-dihydrothieno[2,3-c]pyridine-3,6(5H)-dicarboxylate (intermediate 1, 0.5 g, 1.60mmol) in a...
Embodiment 2
[0057] Example 2: 6-Benzyl-2-(((4-methylphenyl)sulfonamido)-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxy Synthesis of methyl esters:
[0058] With reference to the synthetic method of Example 1, the intermediate 2 in Example 1 is replaced by 6-(tert-butyl) 3-methyl 2-(((4-methylphenyl)sulfonyl)-4,7- Dihydrothieno[2,3-c]pyridine-3,6(5H)-dicarboxylate, a yellow solid compound, namely 6-benzyl-2-(((4-methylphenyl)sulfonyl Amino)-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylic acid methyl ester (compound 2).TLC detects as one point, there is a dark spot under the ultraviolet lamp 254nm, 365nm No fluorescence. 1 H NMR (500MHz, CDCl 3 ): δ7.79(d, J=8.2Hz, 2H), 7.40-7.30(m, 6H), 7.27(s, 1H), 3.79(s, 3H), 3.70(s, 2H), 3.52(s, 2H), 2.79(t, J=5.7Hz, 2H), 2.75(t, J=5.7Hz, 2H), 2.42(s, 3H).HRMS(ESI)m / zcalcd.for C 23 h 24 N 2 o 4 S 2 [M+H] + 456.1177, found 456.1172.
Embodiment 3
[0059] Example 3: 6-Benzyl-2-(((4-(tert-butyl)phenyl)sulfonyl)-4,5,6,7-tetrahydrothieno[2,3-c]pyridine- Synthesis of methyl 3-carboxylate:
[0060] With reference to the synthetic method of Example 1, intermediate 2 in Example 1 is replaced by 6-(tert-butyl) 3-methyl 2-(((4-(tert-butyl)phenyl)sulfonamido)-4 , 7-dihydrothieno[2,3-c]pyridine-3,6(5H)-dicarboxylate, to obtain a yellow solid compound, which is 6-benzyl-2-(((4-(tert-butyl base) phenyl) sulfonamido)-4,5,6,7-tetrahydrothieno[2,3-c]pyridine-3-carboxylic acid methyl ester (compound 3).TLC detects as one point, UV lamp 254nm There are dark spots below and no fluorescence at 365nm. 1 H NMR (500MHz, CDCl 3 ): δ7.93(d, J=8.5Hz, 2H), 7.62(s, 1H), 7.45(d, J=8.5Hz, 2H), 7.41(t, J=6.4Hz, 3H), 7.32-7.29 (m, 2H), 4.75(s, 2H), 3.81(s, 3H), 3.55(t, J=8.3Hz, 2H), 3.26(t, J=8.4Hz, 2H), 1.32(s, 9H) .HRMS(ESI)m / z calcd.for C 26 h 30 N 2 o 4 S 2 [M+H] + 499.1647, found 499.1650.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com