Application of kaempferol 3-glucorhamnoside in preparation of medicine for treating systemic severe sepsis
A technology of bakinosu and sepsis, which is applied in the field of biomedicine to achieve the effect of reducing lung damage, improving and treating severe sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
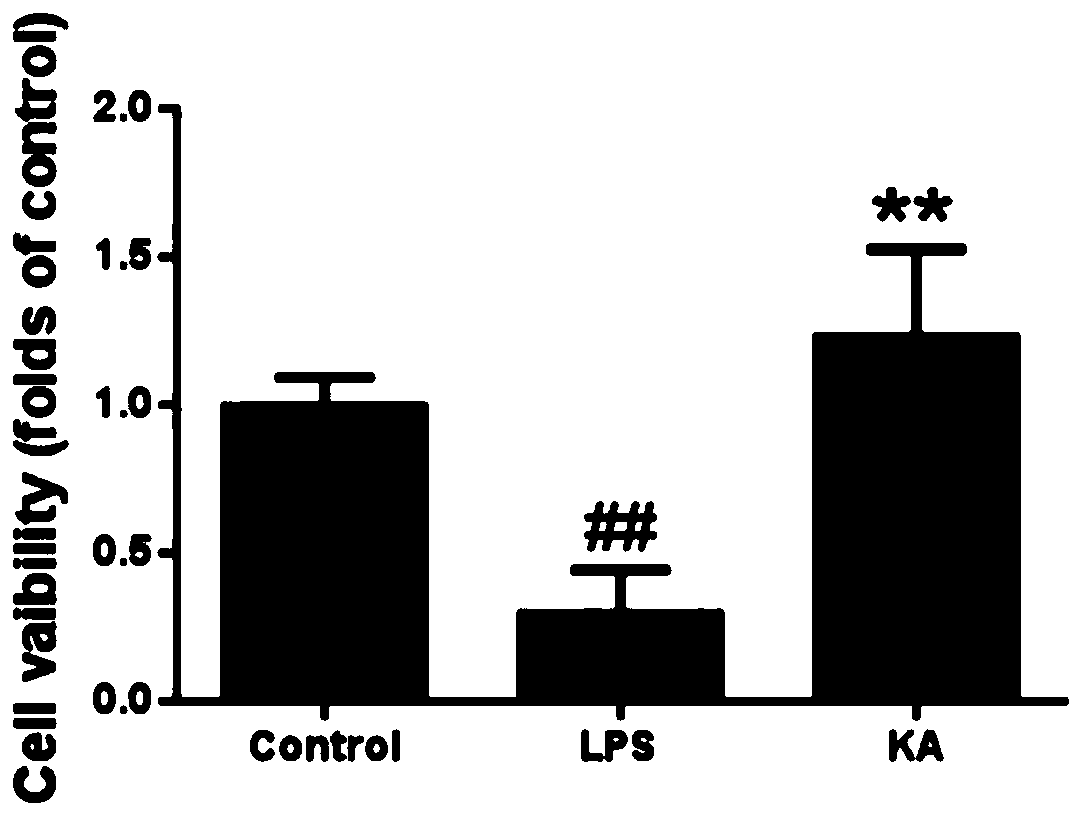
[0037] Effects of thymosin I on cell viability
[0038] experimental method
[0039] Experimental grouping: blank control group, LPS group, thymosin I group
[0040] RAW 264.7 cells were seeded into 96-well plates (2×10 4 Cells / well) were cultured for 24 hours and added with a concentration of 4 μg / ml thymeclidin I, the treatment time was 1 hour, and then exposed to LPS for 18 hours. Subsequently, the 96-well plate was washed twice with PBS, MTT (5 mg / ml) was added to the cells, and incubated for another 4 hours. Add DMSO to dissolve, and measure the absorbance at 570nm; the blank control group is not subjected to any treatment, and the LPS group is only treated with LPS, without treatment with thymosin I.
[0041] Experimental results
[0042] Table 1 Effect of thymosin I on cell viability
[0043]
[0044] (All data are expressed as mean ± SEM (n = 5) ## P* P** P<0.01, compared with LPS group. )
[0045] The cell viability of RAW264.7 cells treated with thymosin I...
Embodiment 2
[0047] Anti-inflammatory effect of thymosin I on cells
[0048] experimental method
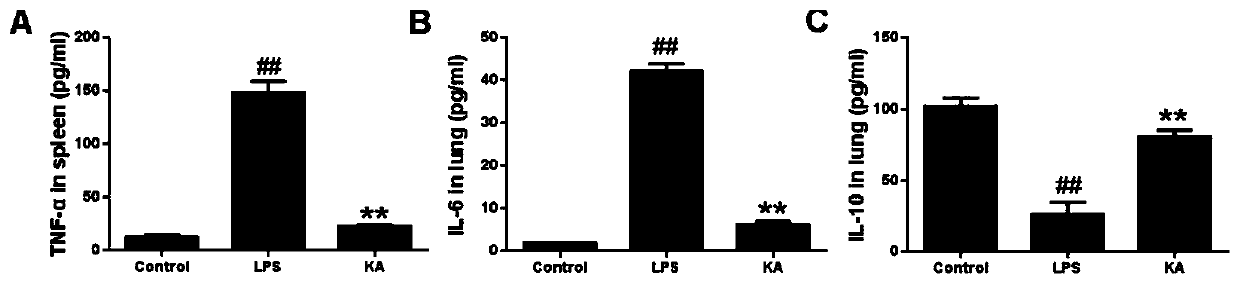
[0049] Experimental grouping and drug treatment were the same as in Example 1, with 5 replicate wells set up for each group. After drug treatment, the cells were placed at 37°C, 5% CO 2 Continuously cultured in the incubator for 72 hours, after that, the cells were blown evenly in a 96-well plate, the cell suspension was collected, centrifuged (3000r / min, 20min), and the cell supernatant was collected. The operation steps were carried out according to the instructions of the ELISA detection kit. Content of TNF-α, IL-10, IL-6.
[0050] Experimental results
[0051] Table 2 The effect of thymosin I on the secretion of inflammatory cytokines in LPS-challenged RAW264.7 cells
[0052]
[0053]
[0054] (All data are expressed as mean ± SEM (n = 5) ## P* P** P<0.01, compared with LPS group. )
[0055] The contents of TNF-α, IL-6 and IL-10 in RAW264.7 cells treated with thymosin I (4 μg / ml...
Embodiment 3
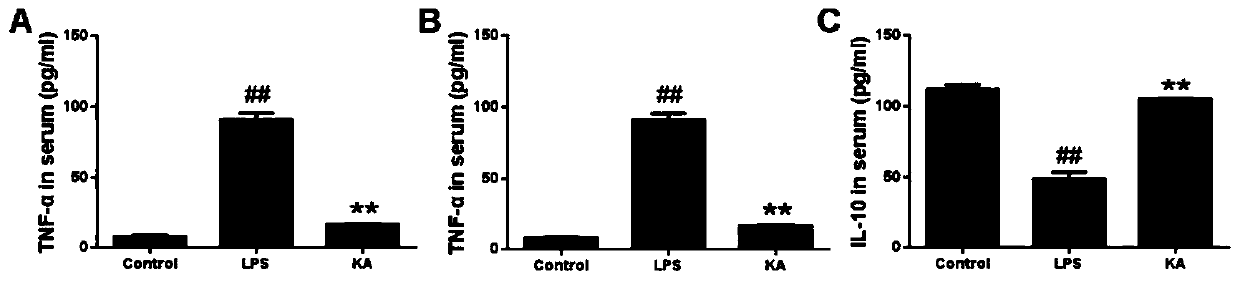
[0057] Effects of thymosin I on TNF-α, IL-10 and IL-6 in mouse serum, liver, spleen and lung
[0058] (1) The effect of thymosin I on the contents of TNF-α, IL-10 and IL-6 in the supernatant of liver homogenate.
[0059] experimental method
[0060] Experimental grouping: blank control group, thymosin I group, LPS group
[0061] 18 mice were randomly divided into blank control group, thymosin I group (200 μg / ml), LPS group (10 mg / kg), 6 mice in each group. 200 μg / ml of thymelin I was injected into the mice of thymelin I, and after 1 hour of treatment, the thymelin I group and the LPS group were challenged; the blank control group did not receive any treatment. Collect the mouse serum, liver, spleen and lungs of each group and make a tissue homogenate suspension, dilute the tissue homogenate suspension 10 times, centrifuge at 3600r / min for 10 minutes, and collect the homogenate supernatant; after that, according to ELISA The test kit instructions are used to detect the level...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com