Application of ozone in treatment of sepsis complications caused by noval coronavirus 2019 infection
A 2019-ncov, coronavirus technology, applied in the field of biomedicine, can solve problems such as not having a good treatment method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
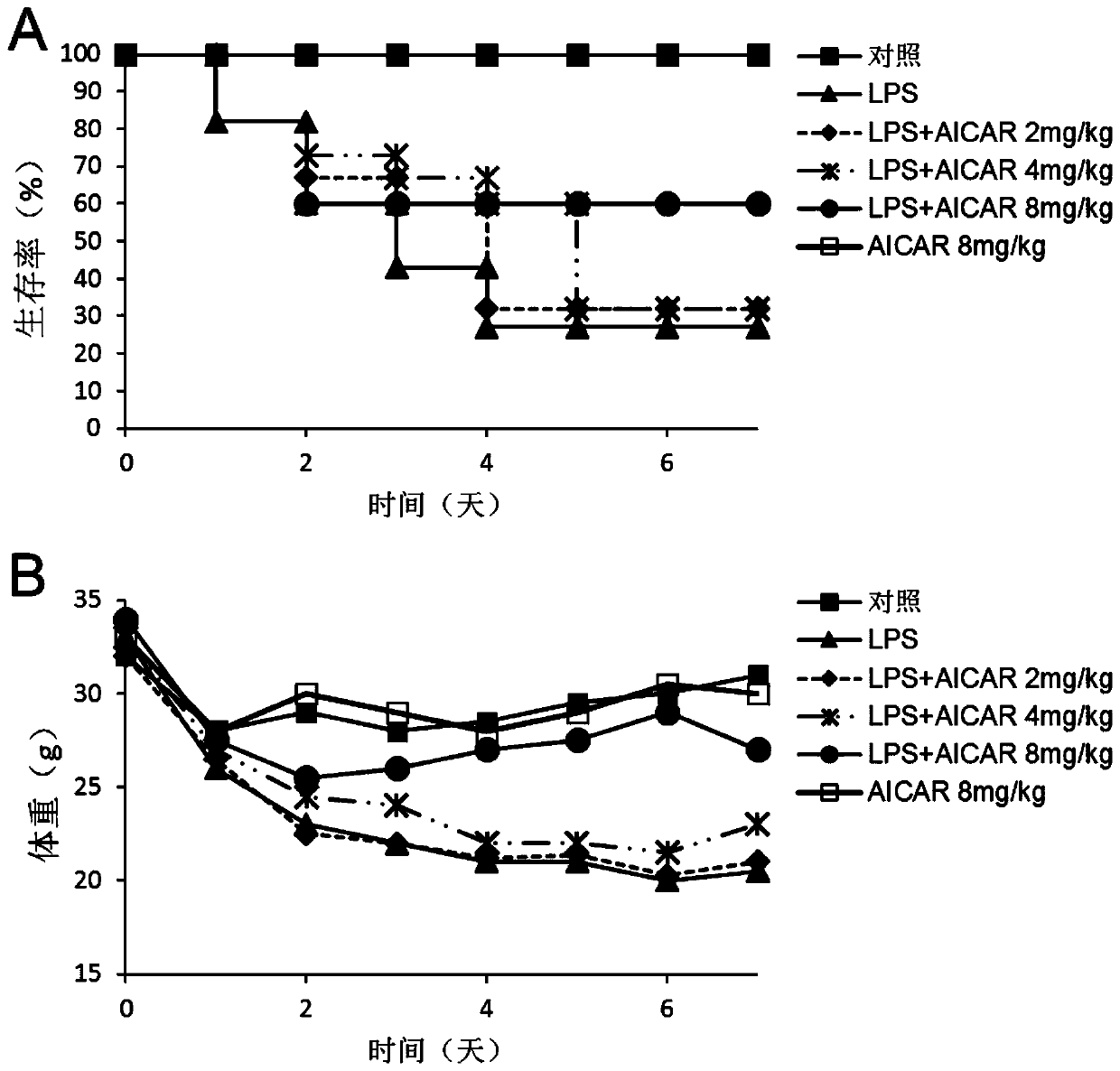
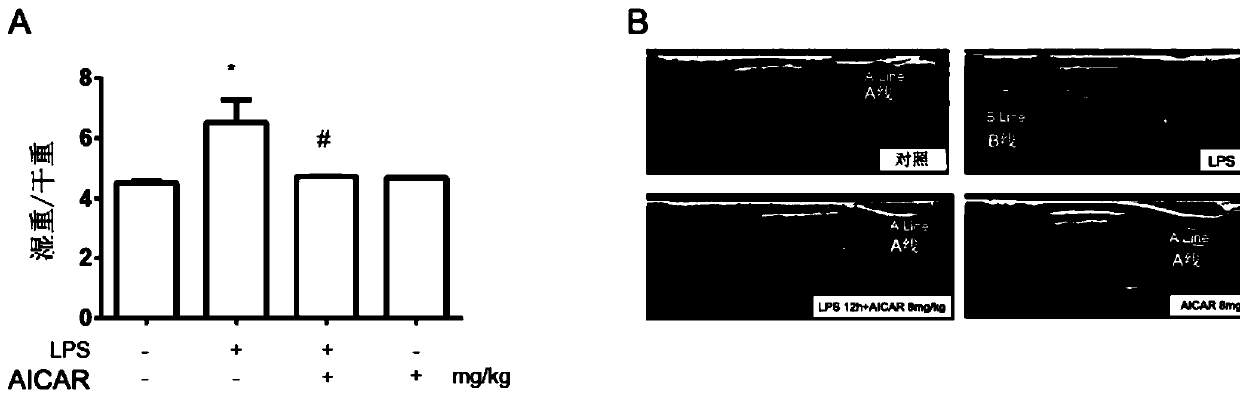
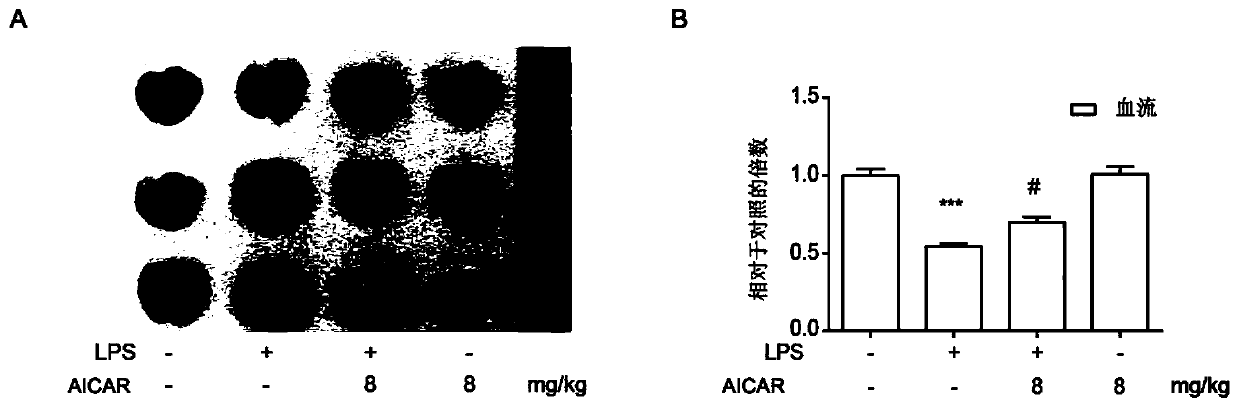
[0043] Example 1 The protective effect of ozone autohemotherapy on lung injury in ALI mice
[0044] 1) Establishment of sepsis lung injury (ALI) model
[0045]C57 mice 4-6 weeks old, male, were fed with standard diet for 3 days, weighed the mouse balance, anesthetized by intraperitoneal injection of 5% chloral hydrate (1mg / mL), and injected LPS 5mg / kg through the tail vein to establish the machine. model of lung injury. The control group was injected with the same volume of normal saline. The arterial partial pressure of oxygen (PaO 2 ), lung index (LI), lung permeability index (LPI), and observed the mRNA levels of inflammatory factors IL-β, TNF-α and IL-6 in the lung tissue of mice, which had significant statistical significance, P<0.05 . At the same time, pathological light microscopy was performed on the lung tissue by HE staining to confirm that the ALI modeling was successful.
[0046] 2) The protective effect of AMPK activation on ALI lung injury
[0047] AMPK ago...
Embodiment 2
[0081] Embodiment 2 ozone treatment COVID-19 sepsis lung injury
[0082] 1) Experimental design
[0083] The present embodiment adopts the randomized and controlled research method to compare the effectiveness and safety of the test ozone autohemotherapy on the viral pneumonia caused by the COVID-19 virus. Before conducting this trial, the trial protocol, the proposed informed consent form, and other information provided to the patients were submitted to the Institutional Ethics Committee (IEC) for review.
[0084] According to the relevant provisions of Guoyaojianzhu [2002] No. 437 and the "Measures for the Administration of Drug Registration" (Trial Implementation), a total of 20 pairs of clinical research should be completed (20 cases in the test group and 20 cases in the control group).
[0085] 2) Subject population
[0086] 2.1 Inclusion criteria
[0087] (1) COVID-19 confirmed patients infected with 2019-new coronavirus;
[0088] (2) Age 18-65 years old, gender is not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com