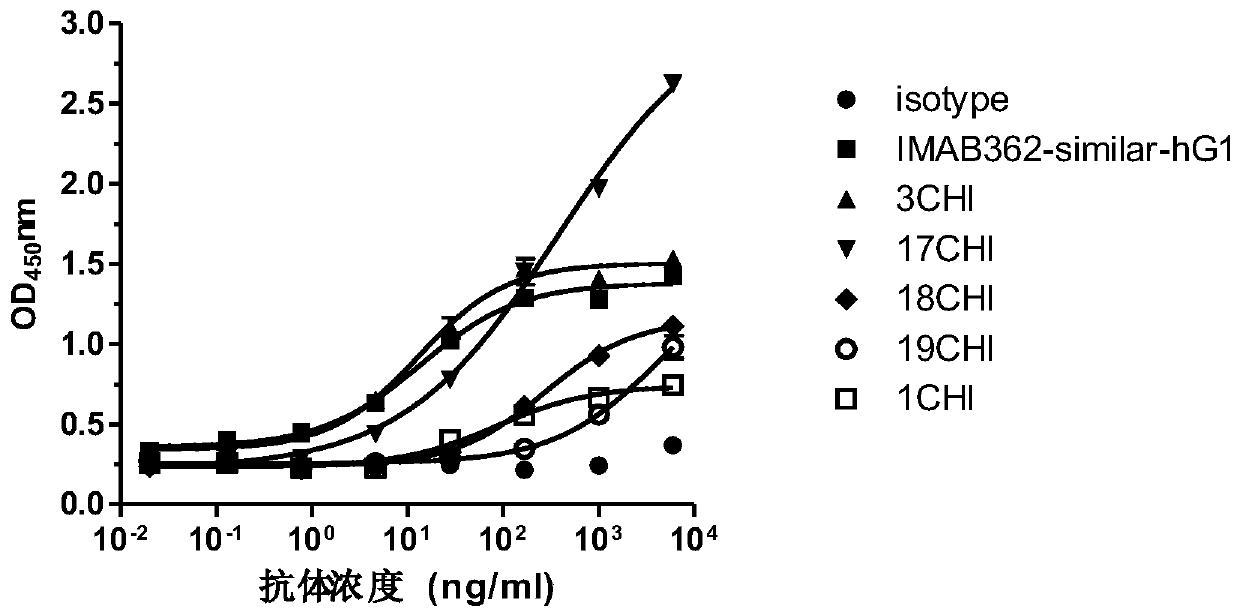
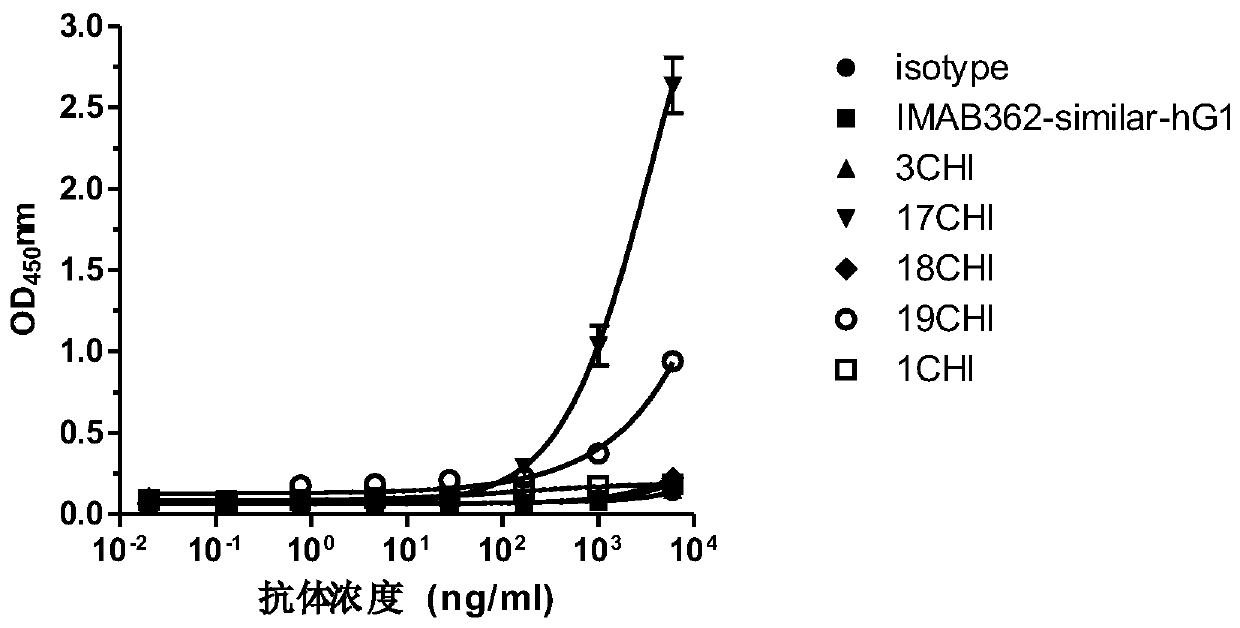
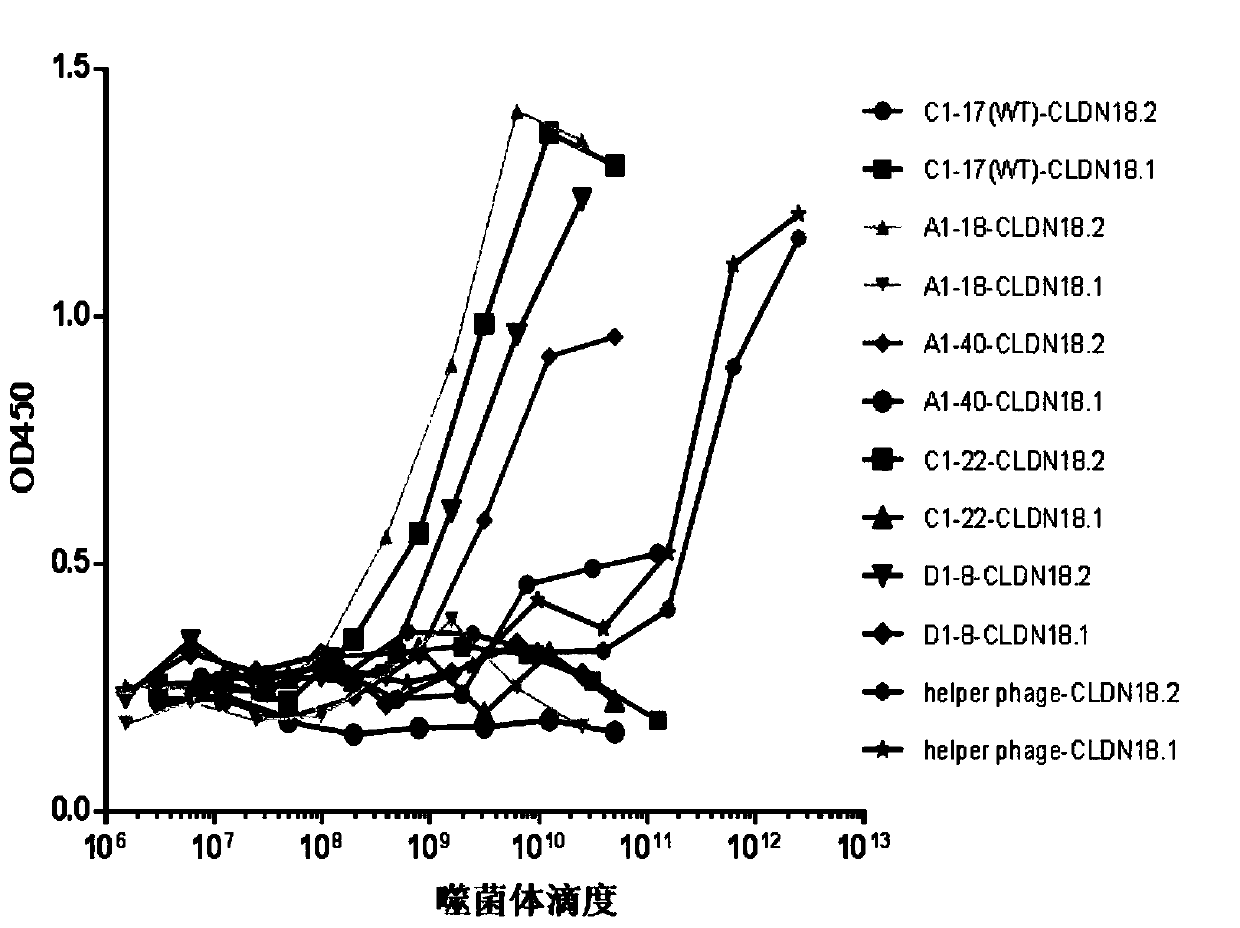
Anti-Claudin 18_2 antibody and application thereof
An antibody and antigen technology, applied in the fields of application, antibody, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problem of loose structure, incomplete protein glycosylation, and easy invasion and metastasis of gastric cancer cells And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] The preparation of embodiment 1 positive control antibody
[0112] 1. Preparation of positive control antibody IMAB362-similar-hG1:
[0113] According to the 175D10 clone (IMAB362) antibody sequence (ie, SEQ ID NO 11 and SEQ ID NO 12 in this disclosure) provided by the patent application WO2007 / 059997, Nanjing GenScript Co., Ltd. was entrusted to perform codon optimization and then synthesized in pcDNA3. 1(+) vector. The constructed plasmids include pcDNA3.1-hG1 and pcDNA3.1-hK. The plasmid was co-transfected into expiCHO cells at a ratio of 1:1 for transient expression at 110 rpm, 37°C, 8% CO 2 After culturing for 4 days, transfer to a shaker at 32°C to continue culturing for 8-10 days. Centrifuge at 4500rpm for 25 minutes, harvest the protein supernatant and purify by ProteinA affinity chromatography: wash the AKTA chromatography system and chromatography column with 0.1M NaOH solution for 30min, rinse with ultrapure water; column volume; set the flow rate at 2ml / ...
Embodiment 2
[0118] Example 2 CLDN18.2 antigen gene synthesis and expression vector construction
[0119] The fusion protein sequence design expressing human CLDN18.2 antigen is shown in Table 1 below:
[0120] Table 1 CLDN18.2 sequence design
[0121]
[0122] The fusion protein sequence of the human CLDN18.2 antigen designed above was optimized for eukaryotic codons, and a restriction site such as NotI and tPA signal peptide sequence were added to the 5' end of the gene, and a restriction site such as XhoI was added to the 3' end of the gene and a Myc-His fusion protein tag. The above sequences of SEQ ID NO 1, 3, 5, and 6 were synthesized and constructed into the pcDNA3.1(+) vector to obtain a DNA plasmid expressing human CLDN18.2 antigen. Plasmids include: pcDNA3.1-loop1, pcDNA3.1-TML, pcDNA3.1-CpG-loop1 and pcDNA3.1-CLDN18.2-FL.
[0123] The aforementioned sequences of SEQ ID NO 7 and 9 were optimized and synthesized by Treasure Bioengineering (Dalian) Co., Ltd. (hereinafter refe...
Embodiment 3
[0125] Example 3 Expression of CLDN18.2 antigenic protein
[0126] 1. Transient transfection of plasmid DNA in vitro
[0127] The plasmid amplified and cultured in Example 2 was transiently transfected into HEK293T cells in vitro (Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, hereinafter referred to as Shanghai Cell Institute), and identified at the protein level. The specific experimental steps are as follows: the day before transfection, press 3×10 5 piece / cm 2 The seeding density of HEK293T cells was spread in six-well plates, cultured (medium composition: 10% fetal bovine serum DMEM medium + 1% penicillin-streptomycin) overnight, so that the cells were completely attached on the day of transfection, and Make the cell abundance reach 70%-90% (the size of each hole in a six-well plate is 9cm 2 , cells overgrow about 2-2.5×10 6 per well); use PEI cationic transfection reagent (sigma) or liposome (Lipo3000, Thermo) to mix with DNA plasmid to prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
| Equilibrium dissociation constant | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com