Aromatic acid DDMP diester sweetening agents as well as preparation method and application thereof
A technology for aromatic acids and sweeteners, which is applied in the field of tobacco flavor synthesis, and can solve the problems of poor stability, inability to use tobacco sweeteners, and easy deterioration and damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of above-mentioned aromatic acid DDMP diester comprises the following steps:
[0030] Wherein M is an acylating reagent, specifically
[0031] said Any of benzoyl chloride, m-toluoyl chloride, o-methoxybenzoyl chloride, phenylacetyl chloride, cinnamoyl chloride and p-hydroxycinnamoyl chloride.
[0032] said For benzoic anhydride.
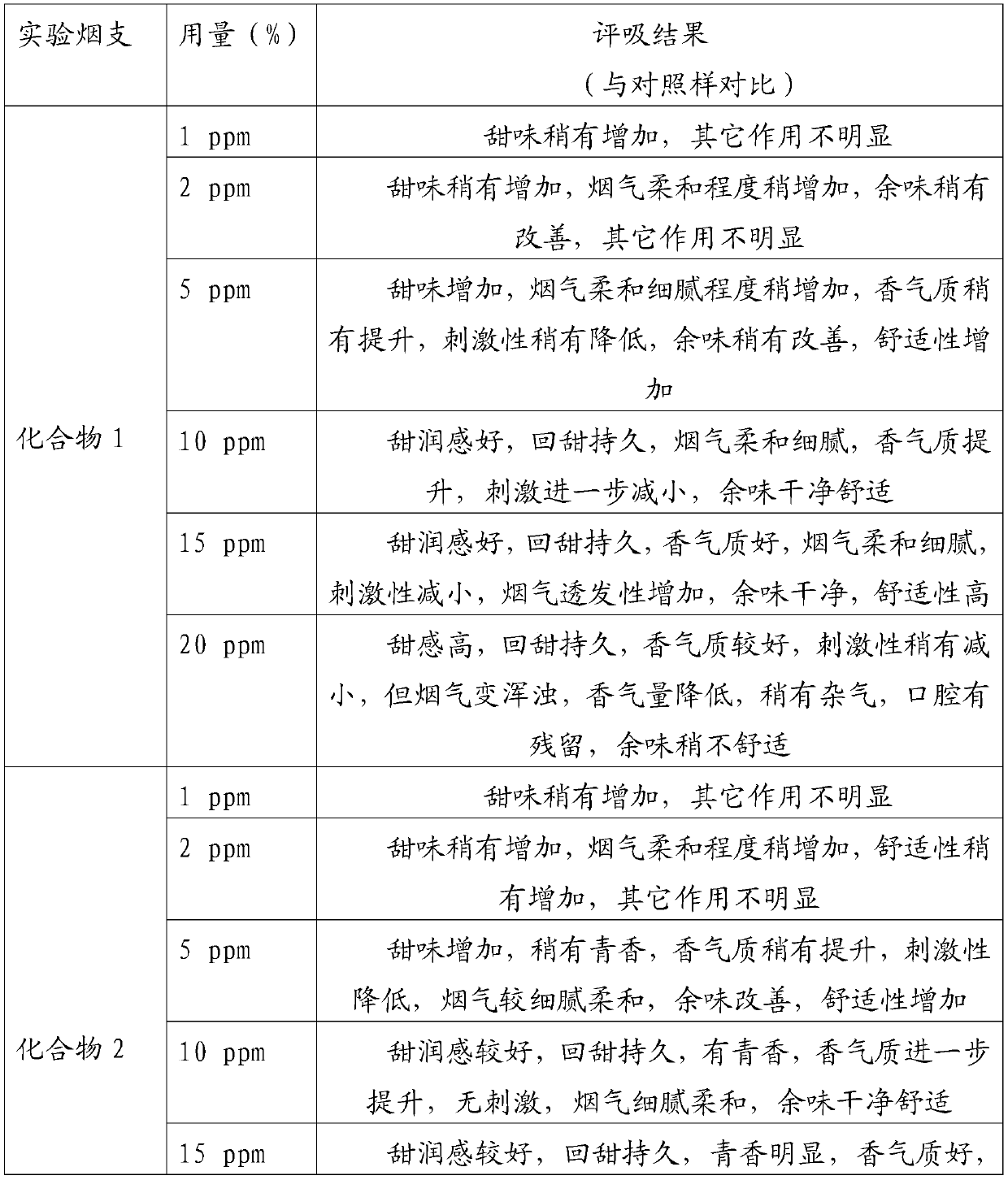
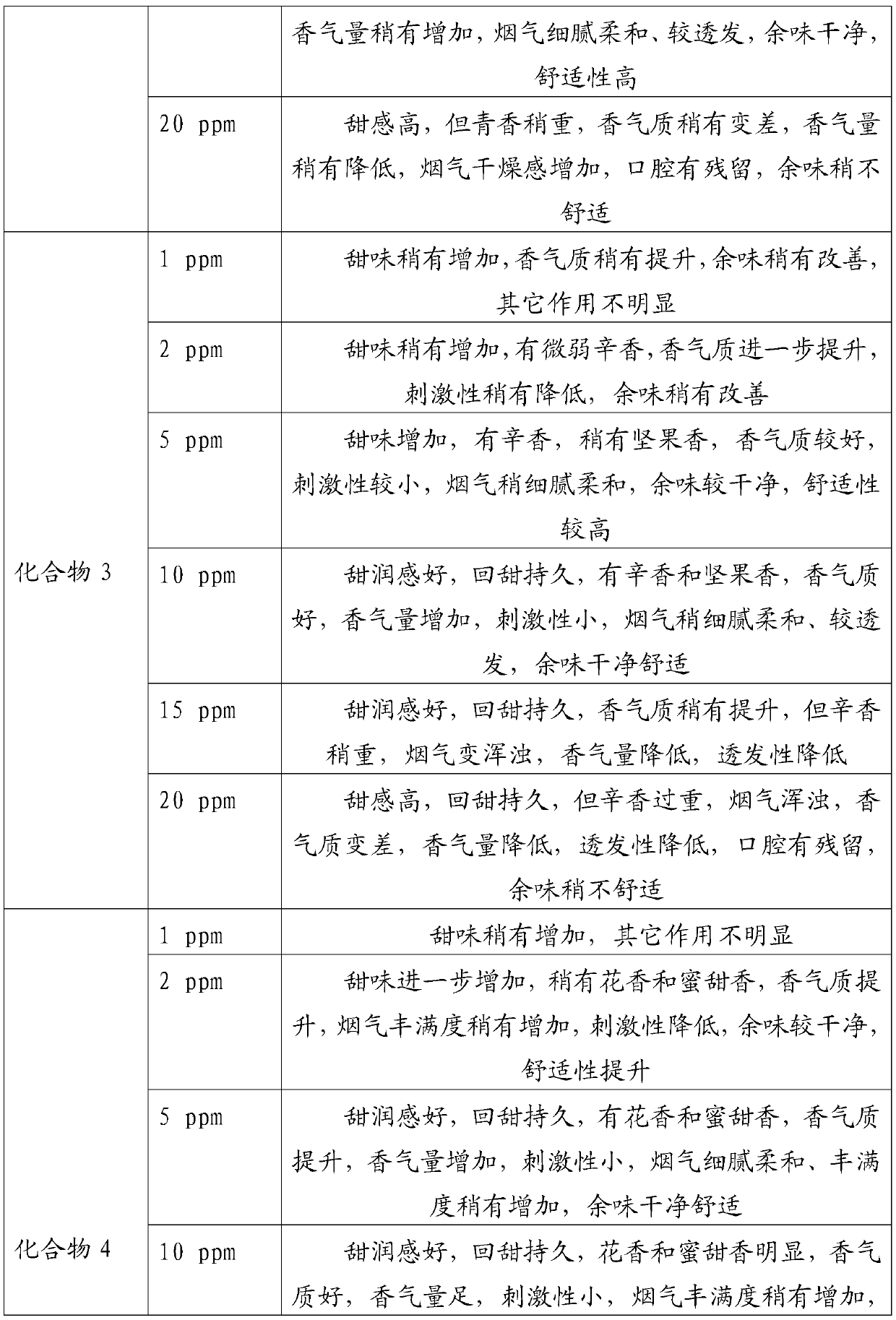
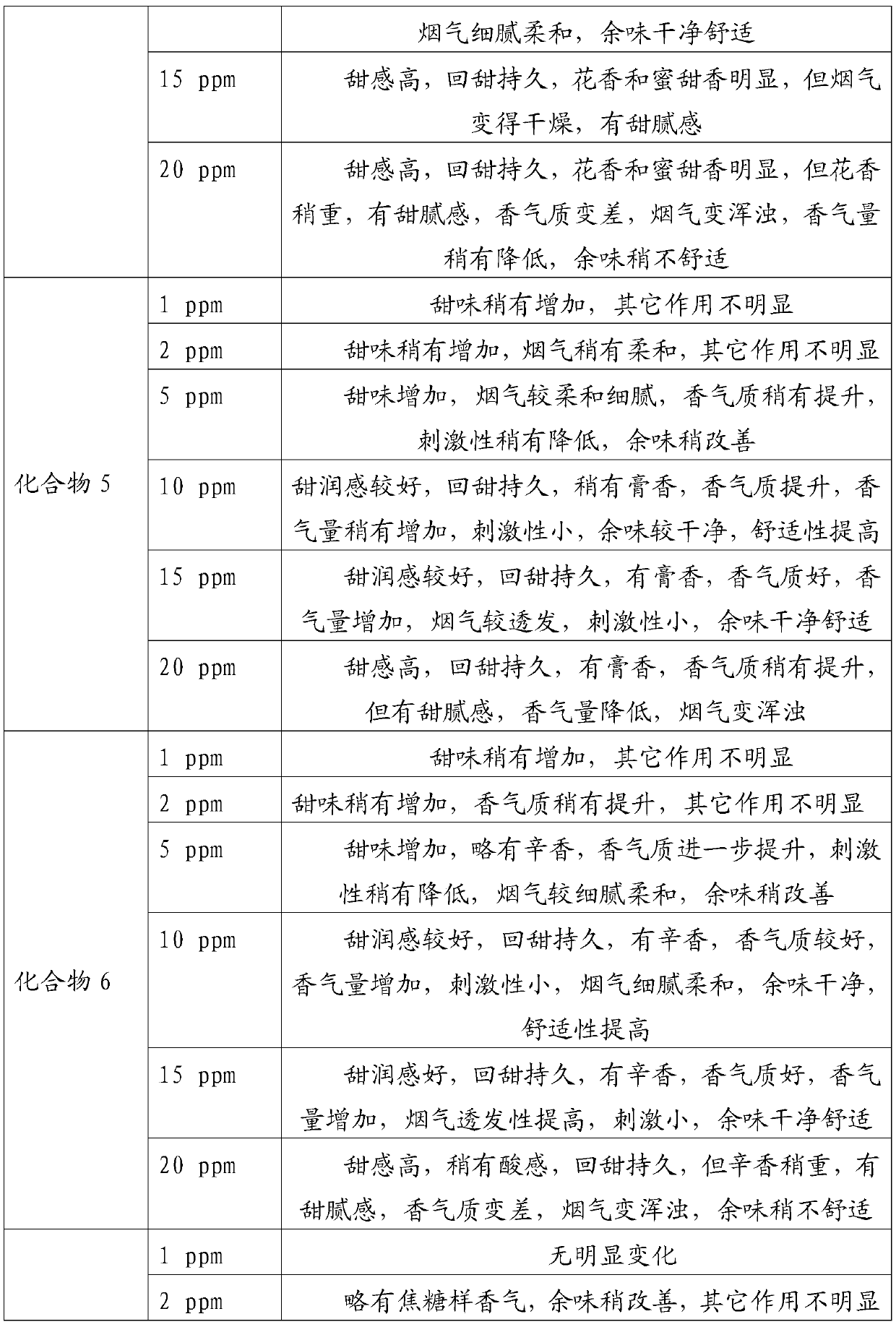
Embodiment 1
[0034] In a 100mL round bottom flask, add 2.88g 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (20mmol) and 40mL of anhydrous dichloromethane, cool To 0 ℃, under the protection of nitrogen, add 4.44g triethylamine (44mmol) and 6.18g benzoyl chloride (44mmol) successively, then rise to room temperature and react for 6h, after the reaction is completed, the reaction solution is washed with water and saturated sodium chloride solution successively 3 times, the organic phase was dried with anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure (in this embodiment and the following embodiments, the vacuum degree of reduced pressure is determined according to actual conditions and needs, as long as the effect of evaporation under reduced pressure is realized That is, it will not affect the realization of the technical solution due to the difference in vacuum), the crude product is separated and purified by silica gel column chromatography, petroleum ether ...
Embodiment 2
[0036] In a 100mL round bottom flask, add 2.88g 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (20mmol) and 40mL anhydrous toluene, cool to - 5°C, under the protection of nitrogen, add 3.56g pyridine (45mmol) and 10.18g benzoic anhydride (45mmol) successively, then rise to room temperature and react for 8h. The organic phase was dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The crude product was separated and purified by silica gel column chromatography, eluting with petroleum ether (V): ethyl acetate (V) = 5:1 to obtain 6.23 g of a white solid. It is 2,3-dihydro-3,5-diacetoxy-6-methyl-4H-pyran-4-one, and the yield is 88.49%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com