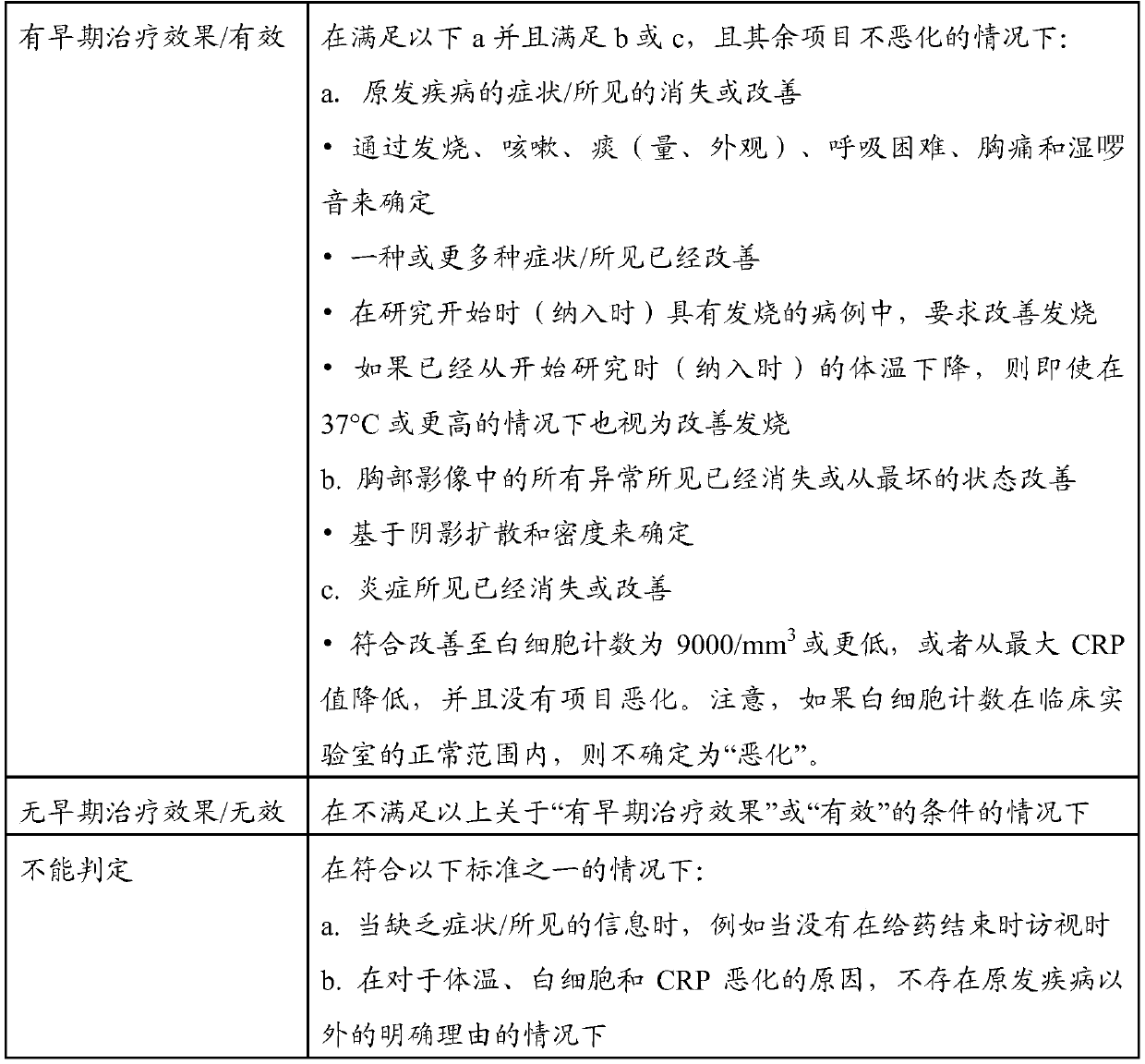
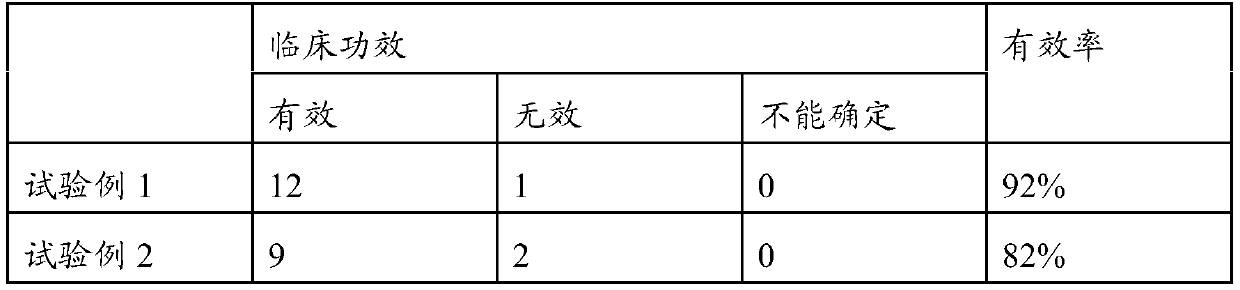
Therapeutic agent for aspiration pneumonia, lung suppuration, or lung abscess
A therapeutic agent, inhalation technology, used in the field of therapeutic agents for aspiration pneumonia, pulmonary suppuration or lung abscess
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0091] Hereinafter, the present invention will be described in more detail by showing examples, but the scope of the present invention is not limited by these examples.
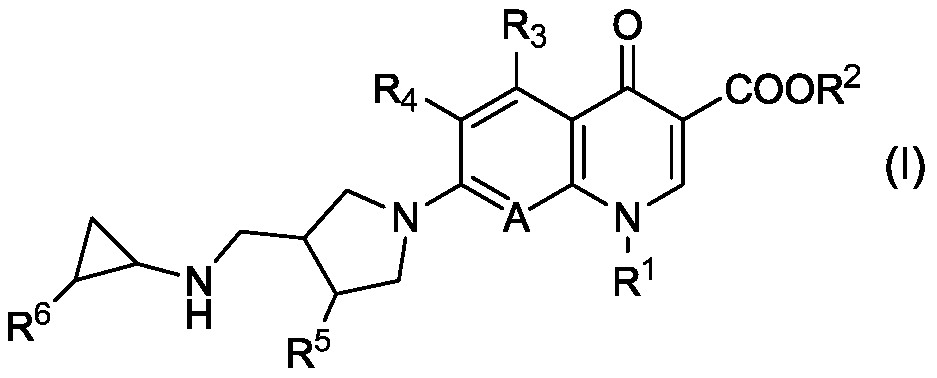
[0092] According to the method disclosed in International Publication No. WO 2016 / 195014, 7-[(3S,4S)-3-{(cyclopropylamino)methyl}-4-fluoropyrrolidin-1-yl]-6 - 150 mg injection of fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (hereinafter also referred to as Investigational New Drug A) .
[0093] "150mg" in 150mg injection indicates that in 7-[(3S,4S)-3-{(cyclopropylamino)methyl}-4-fluoropyrrolidin-1-yl]-6-fluoro-1-( 2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride is the weight in terms of free form. In the preparation of injections, 162.5 mg (converted to free form: 150 mg) of 7-[(3S,4S)-3-{(cyclopropylamino)methyl}-4-fluoropyrrolidin-1-yl]- 6-Fluoro-1-(2-fluoroethyl)-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylate hydrochloride. (Te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com