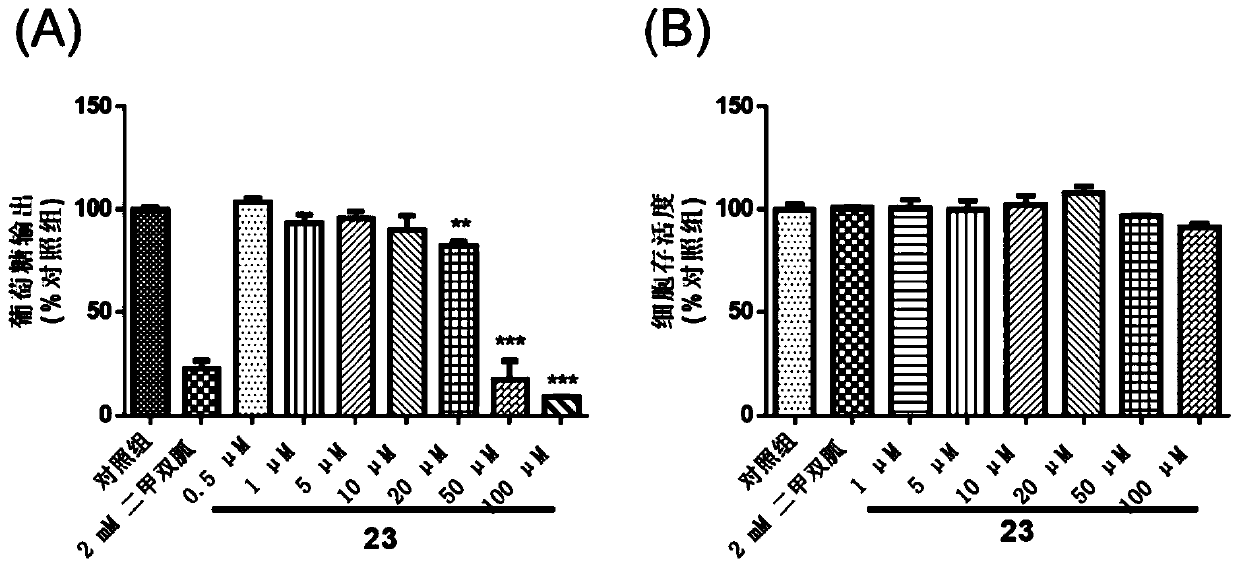
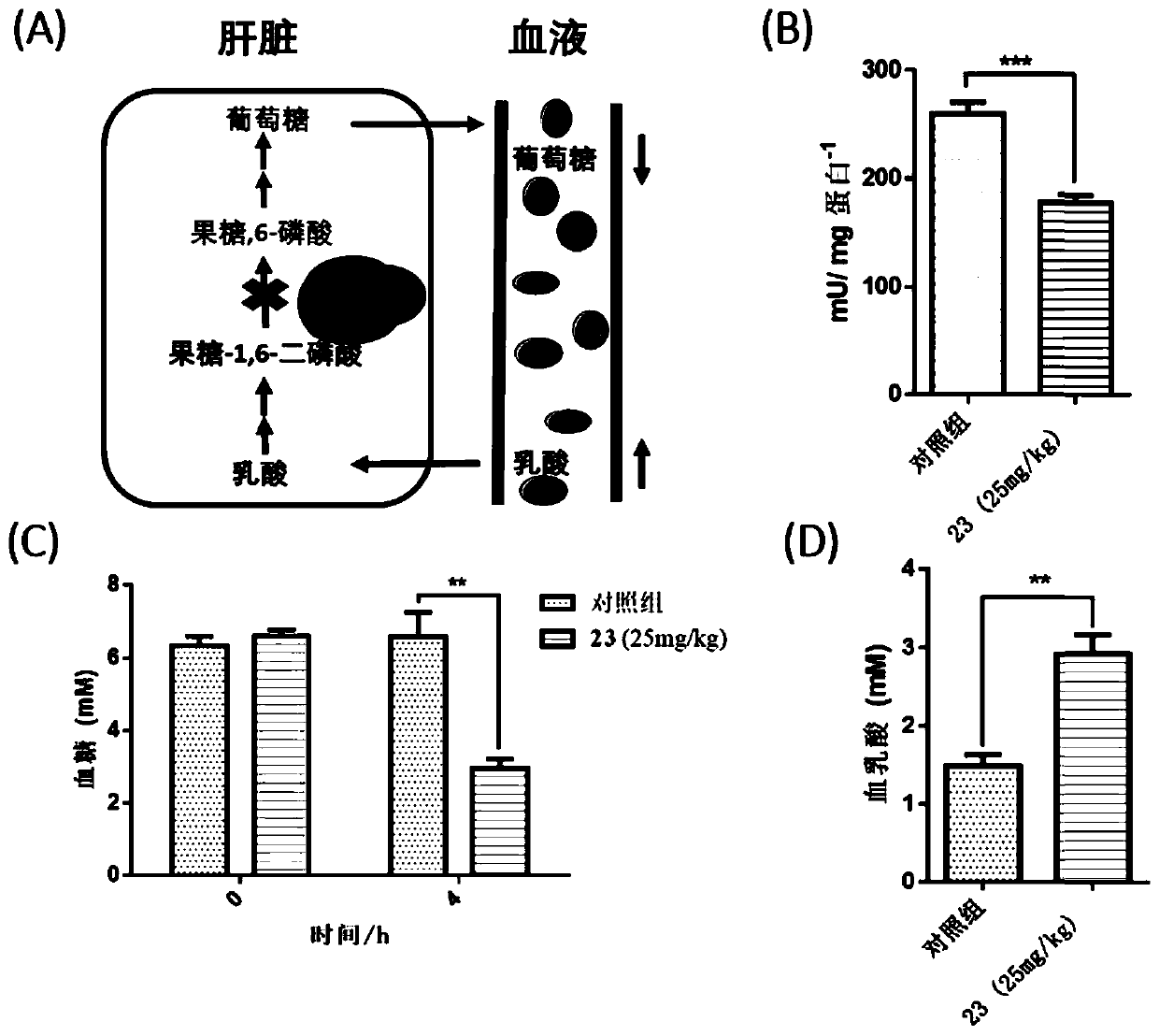
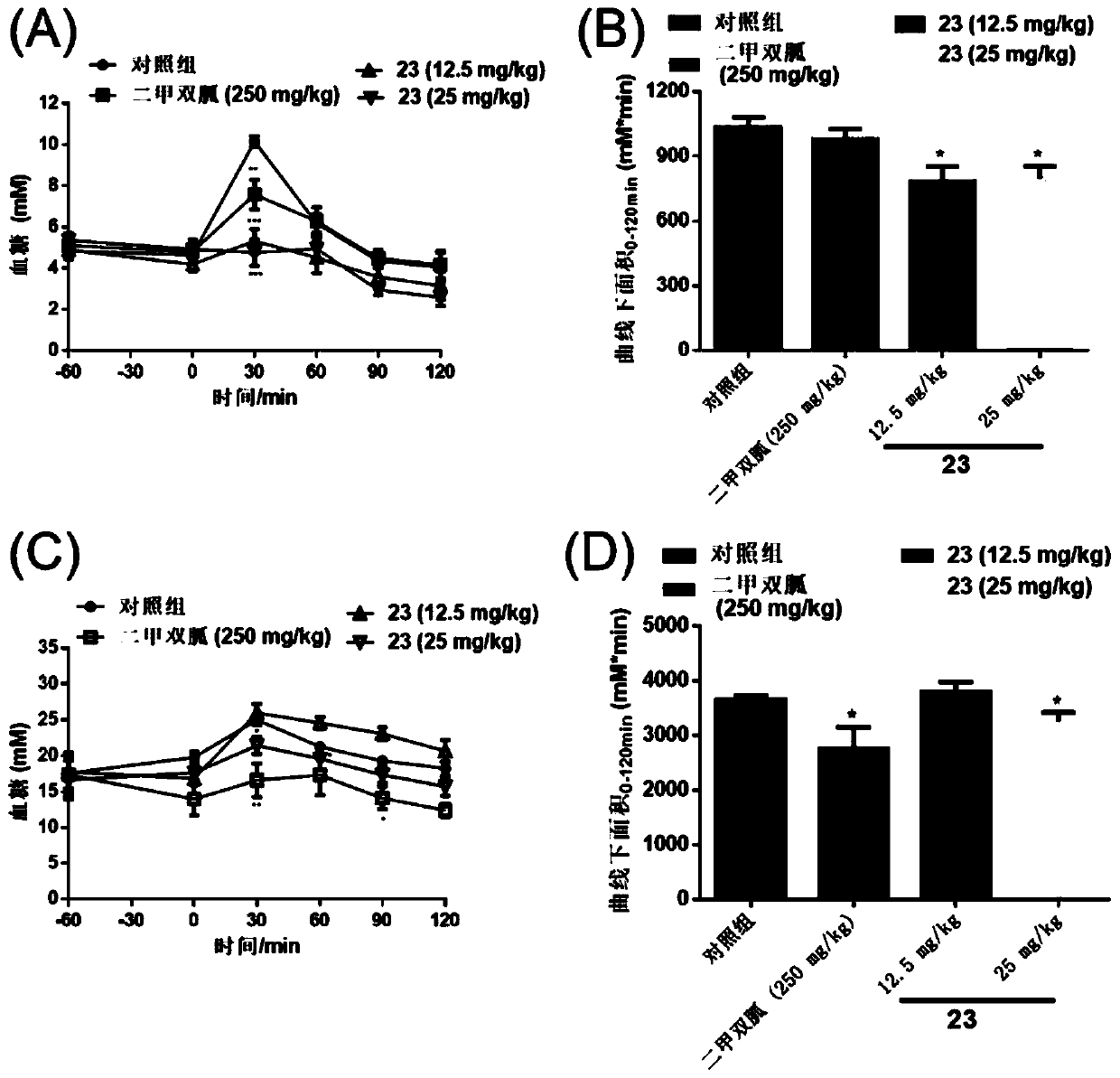
Application of disulfide compound in preparation of medicines, FBP enzyme inhibitor, and medicine for preventing and/or treating diabetes
A technology of disulfide compounds and compounds, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as poor pharmacokinetic properties and difficulty in developing inhibitors, and achieve excellent gluconeogenesis inhibition and hypoglycemic efficacy and cost The effect of low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0038] In formula (X), R a and R b each independently selected from substituted or unsubstituted C 1-30 Alkyl, substituted or unsubstituted C 3-20 Cycloalkyl, substituted or unsubstituted C 6-20 Aryl and substituted or unsubstituted C 2-20 A heteroaryl group containing at least one heteroatom selected from S, O and N in the heteroaryl group;
[0039] R a and R b The optional substituents in each independently selected from C 1-6 Alkyl, phenyl, C 1-6 Carboxyl, halogen, amino, C 1-6 Alkoxy, nitro, hydroxyl, C substituted by 1-3 halogen 1-6 at least one of the alkyl groups.
[0040] In the present invention, unless otherwise specified, "C 1-30 "Alkyl" means an alkyl group with a total number of carbon atoms of 1-30, including straight-chain alkyl groups and branched-chain alkyl groups, for example, the total number of carbon atoms can be 1, 2, 3, 4, 5, 6, 7, 8, 9 , 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30 straight chain alkyl,...
specific Embodiment approach 2
[0047] In formula (X), R a and R b each independently selected from substituted or unsubstituted C 1-30 Alkyl, substituted or unsubstituted C 3-20 Cycloalkyl, substituted or unsubstituted C 6-20 Aryl, substituted or unsubstituted C 2-20 The heteroaryl group and the group shown in formula (Y), and R a and R b At least one of them is a group represented by formula (Y);
[0048] In the group represented by the formula (Y), R 1 and R 2 each independently selected from substituted or unsubstituted C 1-20 Alkyl, substituted or unsubstituted C 6-20 Aryl, or R 1 and R 2 Together with the same N atom to which it is attached, a substituted or unsubstituted ring A group is formed;
[0049] The ring A group is selected from saturated monocyclic groups containing at least 1 N atom, unsaturated monocyclic groups containing at least 1 N atom, saturated bicyclic bridged ring groups containing at least 1 N atom, containing at least 1 An unsaturated bicyclic bridging ring group wit...
specific Embodiment approach 3
[0052] The compound shown in the formula (X) is a compound shown in the formula (I),
[0053]
[0054] Among them, in formula (I), R 1 and R 2 each independently selected from substituted or unsubstituted C 1-20 Alkyl, substituted or unsubstituted C 6-20 Aryl, or R 1 and R 2 Together with the same N atom to which it is attached, a substituted or unsubstituted ring A group is formed;
[0055] The ring A group is selected from a saturated ring group containing at least 1 N atom, an unsaturated ring group containing at least 1 N atom, and the ring-forming atoms of the ring A group optionally contain at least one S atom and / or contain at least one O atom;
[0056] R 1 and R 2 The optional substituents in each independently selected from C 1-6 Alkyl, phenyl, C 1-6 at least one of the carboxyl groups.
[0057] Representative groups of unsubstituted ring A groups can be, for example, at least one group provided by the following substances: azetidine, tetrahydropyrrole, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com