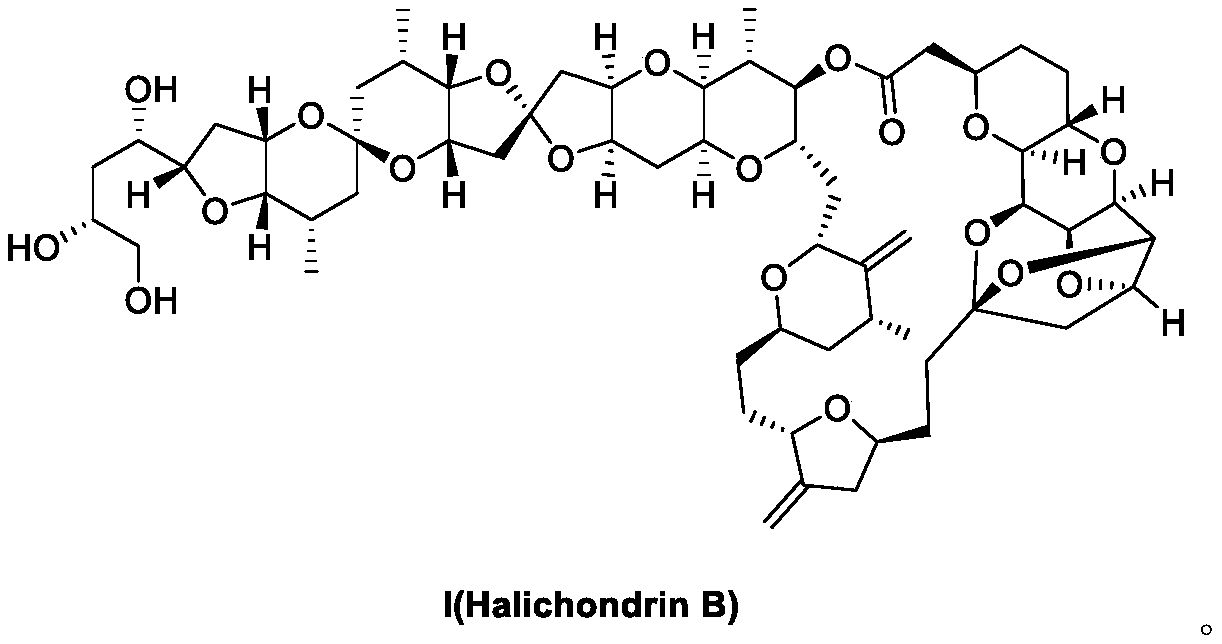
Synthesis method of eribulin intermediate
A synthesis method and intermediate technology, which are applied in the field of pharmaceutical intermediate synthesis, can solve the problems of high cost, poor selectivity, difficult purification and the like, and achieve the effects of simple operation, mild conditions and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
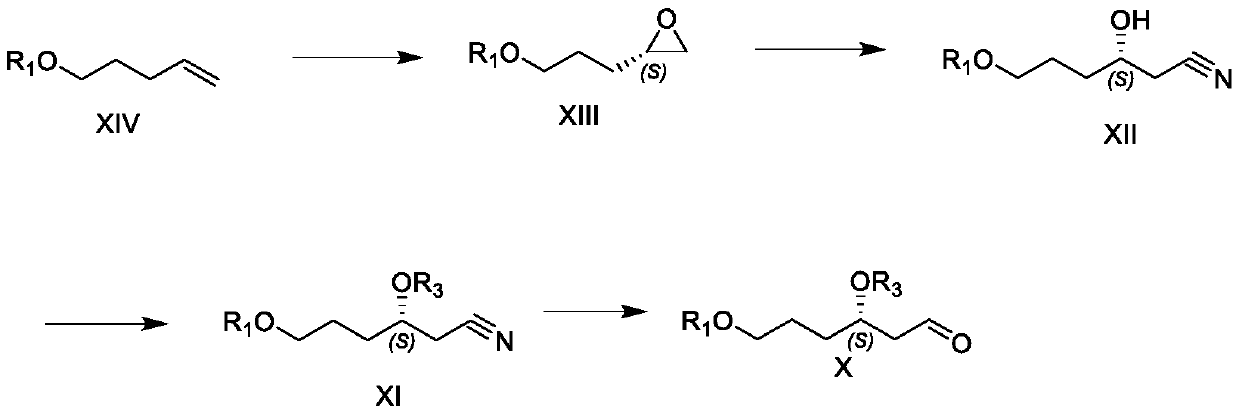
[0066] 1. Preparation of compound XIVa
[0067] Dissolve 4-penten-1-ol (30.0g, 0.35mol) in 150mL DMF, add imidazole (35.6g, 0.52mol), cool down to 0 degrees, and add TBDPSCl (100.5g, 0.365mol) dropwise to the reaction solution . Stir for 1 hour, quench with water, extract with 300 mL of tert-butyl methyl ether, wash with 150 mL of 5% HCl water and 150 mL of saturated brine successively. Concentration gave 110 g of a colorless oily product of formula XIVa, with a yield of 97.3%.
[0068] 1H NMR (400MHz, CDCl3) 7.68-7.66(m, 4H), 7.43-7.35(m, 6H), 5.84-5.75(m, 1H), 4.99(dd, 1H), 4.93(d, 1H), 3.67( t, 2H), 2.17-2.12 (m, 2H), 1.67 (q, 2H), δ1.07 (s, 9H).
[0069] 2. Preparation of Compound XIIIa
[0070] Formula XIVa (25g, 77mmol) was dissolved in 400mL of anhydrous DCM, 30% hydrogen peroxide (460mmol) and Ti-cis-salalen catalyst (23mmol) of 50mL were added, and 30% hydrogen peroxide ( 460mmol), and 50mL of 30% hydrogen peroxide (460mmol) was added again after 24 h...
Embodiment 2
[0099]
[0100] 1. Preparation of compound VIIIb
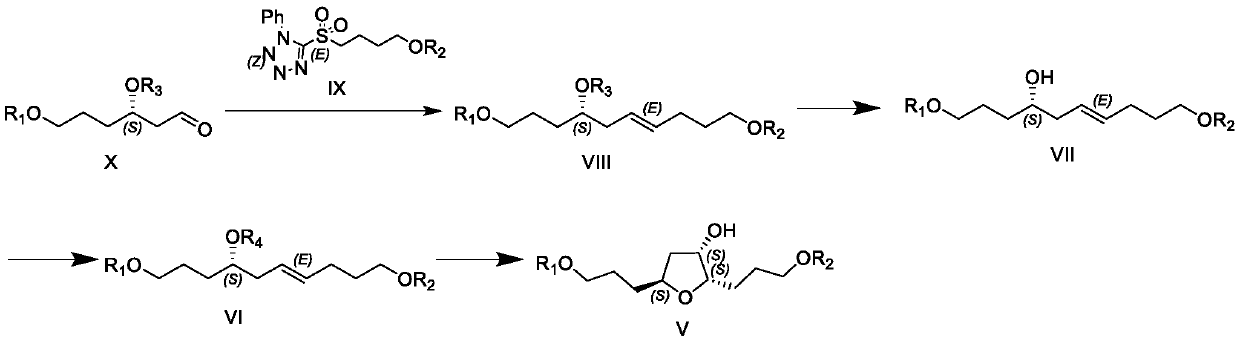
[0101]
[0102] The Julia reagent (28.8g, 77.4mmol) of the compound of formula IXb was added to 200mL of tetrahydrofuran, the temperature was lowered, and the temperature was controlled below -50°C, 77.4mL of 1.0M KHDMS solution in tetrahydrofuran was added dropwise to the reaction flask, and stirred for 15- 20 minutes. Control the temperature below -50°C, add 25.0 g of aldehyde (formula Xa, 51.2 mmol) in tetrahydrofuran (100 mL) dropwise into the reaction flask, and HPLC / TLC detects that the reaction of the raw materials is complete. Add 200 mL of saturated ammonium chloride and 500 mL of tetrahydrofuran, stir for 30 minutes, separate and discard the water layer. The organic layer was washed with 150 mL of saturated sodium chloride water, concentrated, and passed through the column to obtain 28 g of the product (Formula VIIIb), with a yield of 86.0%.
[0103] 1 H-NMR (CDCl-400MHz): 7.69-7.66 (m, 4H), 7.42-7.36 (m, 6H...
Embodiment 3
[0116] Embodiment 3 A kind of synthetic method of Eribulin intermediate B-13
[0117] The synthesis path is as follows:
[0118] (1)
[0119] (2)
[0120] For the specific process of step (1) of this embodiment, refer to [0015] to [0017] in the specification of CN105330686A.
[0121] For the specific process of step (2) of this embodiment, refer to the document J.AM.CHEM.SOC.2009, 131, 15636-15641.
[0122] The compound shown in formula V is synthesized by the synthetic method of Example 1 or Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com