Plasmodium sporozoite NPDP peptides as vaccine and target novel malaria vaccines and antibodies binding to
A Plasmodium and circumsporozoite protein technology, applied in the direction of antibodies, fusion polypeptides, antibody medical components, etc., can solve the problems of not providing significant protection against severe malaria, complicating vaccine development, and reducing efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0605] Example 1: Isolation of human monoclonal antibodies that bind to Plasmodium falciparum sporozoites
[0606] Four malaria-free Tanzanian donors (identified as donors G, H, U and V) were selected for isolation of human monoclonal antibodies. For this purpose, peripheral blood mononuclear cells (PBMC) were isolated from blood samples of four donors. IgG memory B cells were isolated from frozen peripheral mononuclear cells (PBMCs) by magnetic cell sorting. B cells were incubated with 0.5 μg / mL anti-CD19-PECy7 antibody for 20 min on ice, and then with mouse anti-PE microbeads for 30 min on ice. Cells were then stained with 3.75 μg / mL goat Alexa Fluor 647-conjugated anti-human IgG for 20 min on ice and sorted by FACS. Sorted B cells were immortalized with Epstein-Barr (EBV) virus and plated in the presence of CpG and irradiated PBMC feeder cells as previously described in Traggiai et al. (2004) Nat Med. 10, 871-875. Plate in single cell culture. After 14 days, the cultu...
Embodiment 2
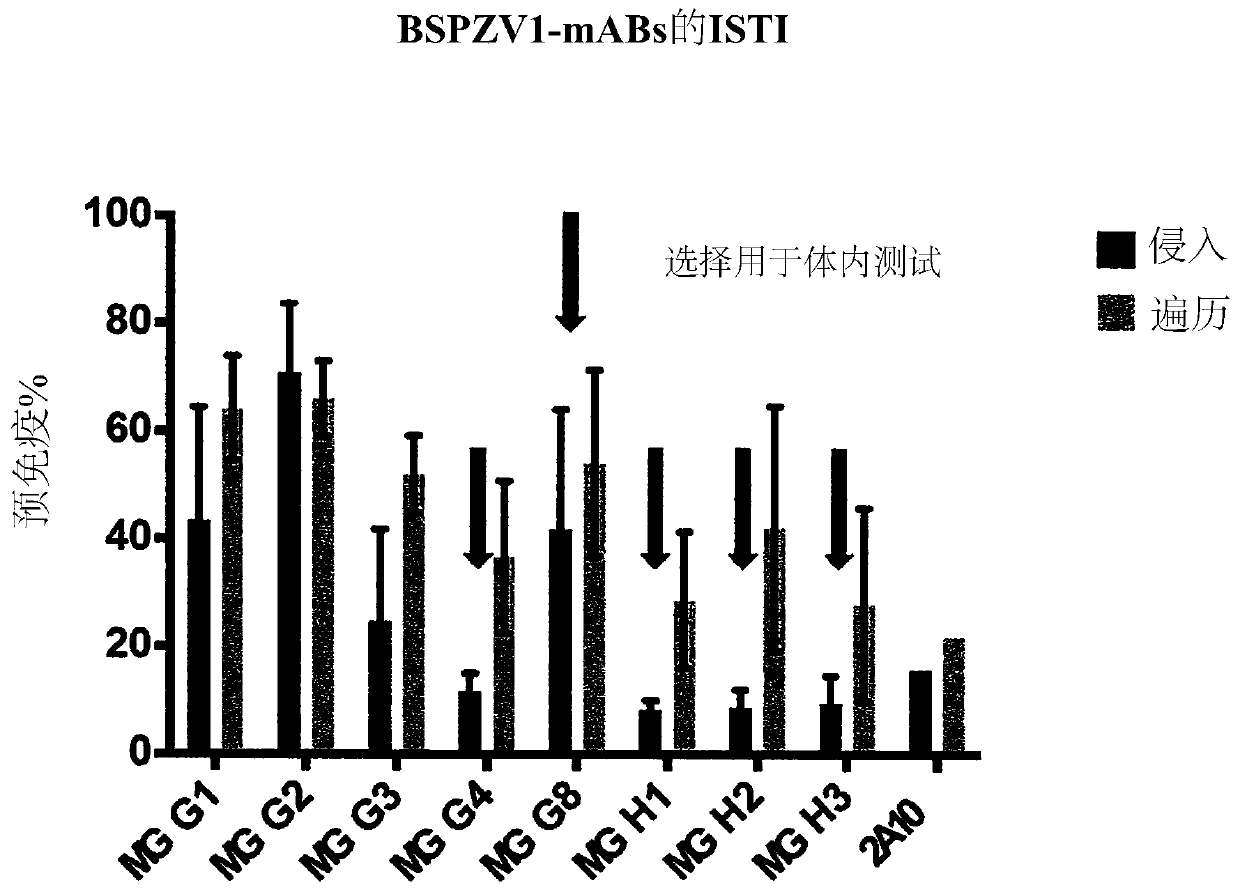
[0612] Example 2: Several monoclonal antibodies show potent anti-sporozoite function in vitro and in vivo
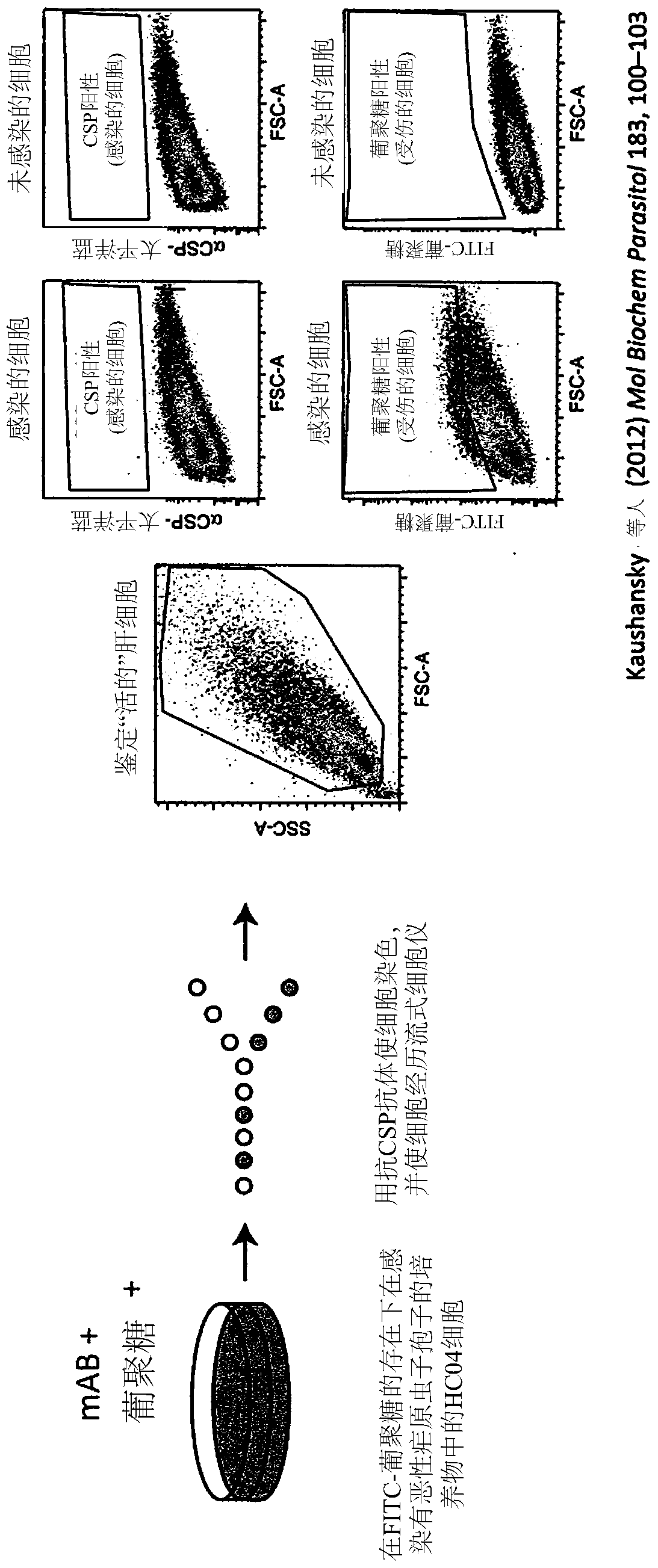
[0613] During the liver stage of the Plasmodium life cycle, sporozoites typically traverse hepatocytes before productively invading target hepatocytes. Exemplary monoclonal antibodies MGG1, MGG2, MGG3, MGG4, MGG8, MGH1, MGH2 and MGH3 (see Tables 1 and 2 for SEQ ID NOs) were tested in vitro for their ability to inhibit sporozoite traversal and invasion of hepatocytes. For this, a quantitative flow cytometry-based assay was used, which is described in Kaushansky A, Rezakhani N, Mann H, Kappe SH, 2012: Development of a quantitative flowcytometry-based assay to assess infection by Plasmodium falciparum sporozoites. Mol Biochem Parasitol.183 (1): 100-3 in. Figure 2A A schematic of this assay is shown. Briefly, in this assay, the hepatocyte HC04 cell line is infected with P. falciparum sporozoites in the presence of FITC-dextran. Sporozoite traversal was measured by upta...
Embodiment 3
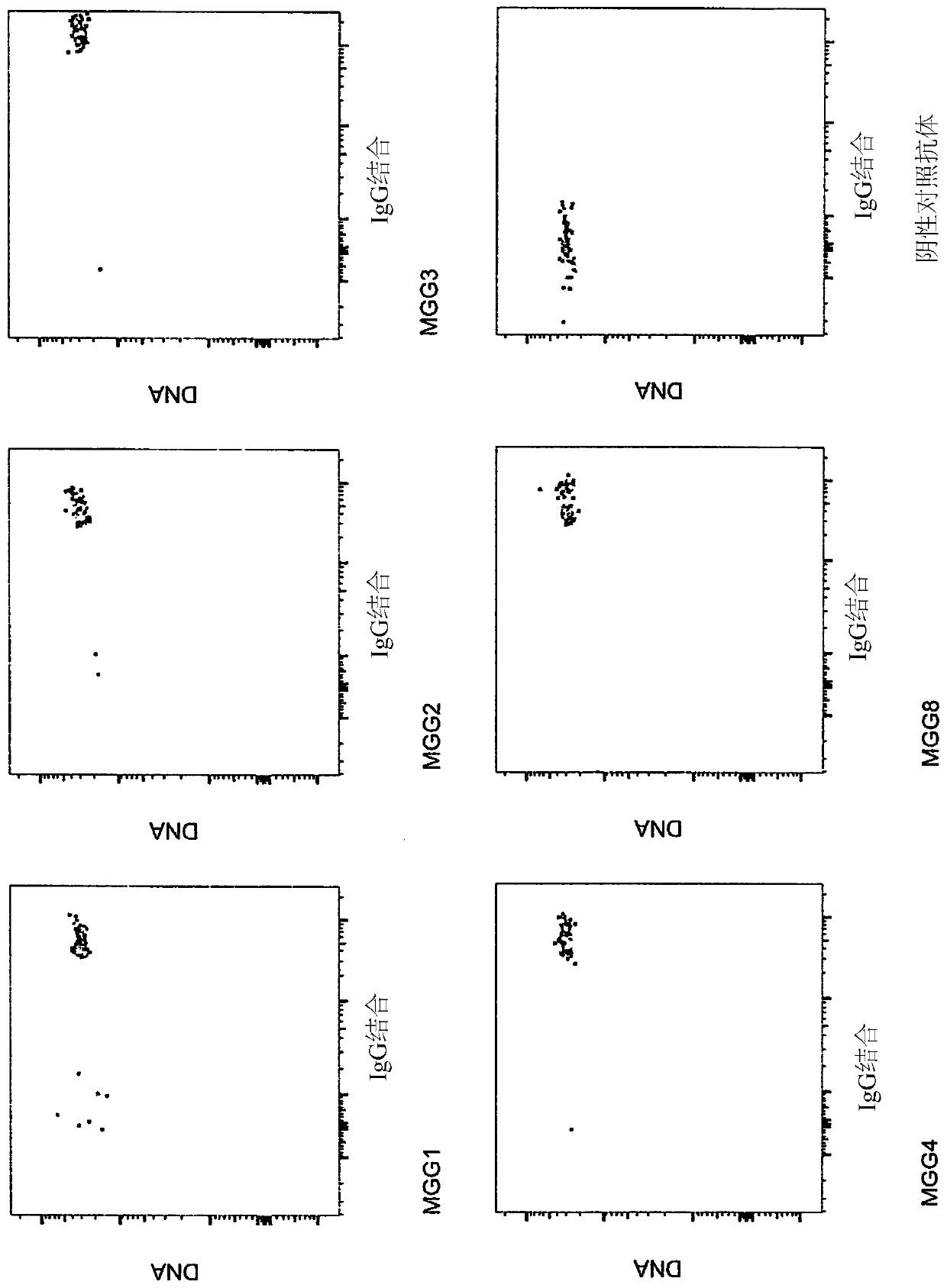
[0617] Example 3: Effective monoclonal antibodies display a unique pattern of binding to CSP and use VH3-30
[0618] Plasmodium circumsporozoite protein (CSP) is an immunodominant protein that coats the entire sporozoite surface and plays an important role in sporozoite function. Such as Figure 4A As shown, this protein contains an N-terminal segment beginning with a signal peptide (SP) and ending with region I (RI). Region I is a pentapeptide (KLKQP; SEQ ID NO:25) involved in binding hepatocytes and mosquito salivary glands. In CSP, region I is followed by the NANP repeat region, the immunodominant site of the antibody and the C-terminal thrombospondin-like domain containing the T cell epitope ( Figure 4A ). Figure 4B An exemplary sequence of the circumsporozoite protein of P. falciparum isolate NF54 is shown (SEQ ID NO: 24).
[0619] An antigen-agnostic approach as described in Example 1 was used to identify any antibodies that could bind to the sporozoite surface. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com